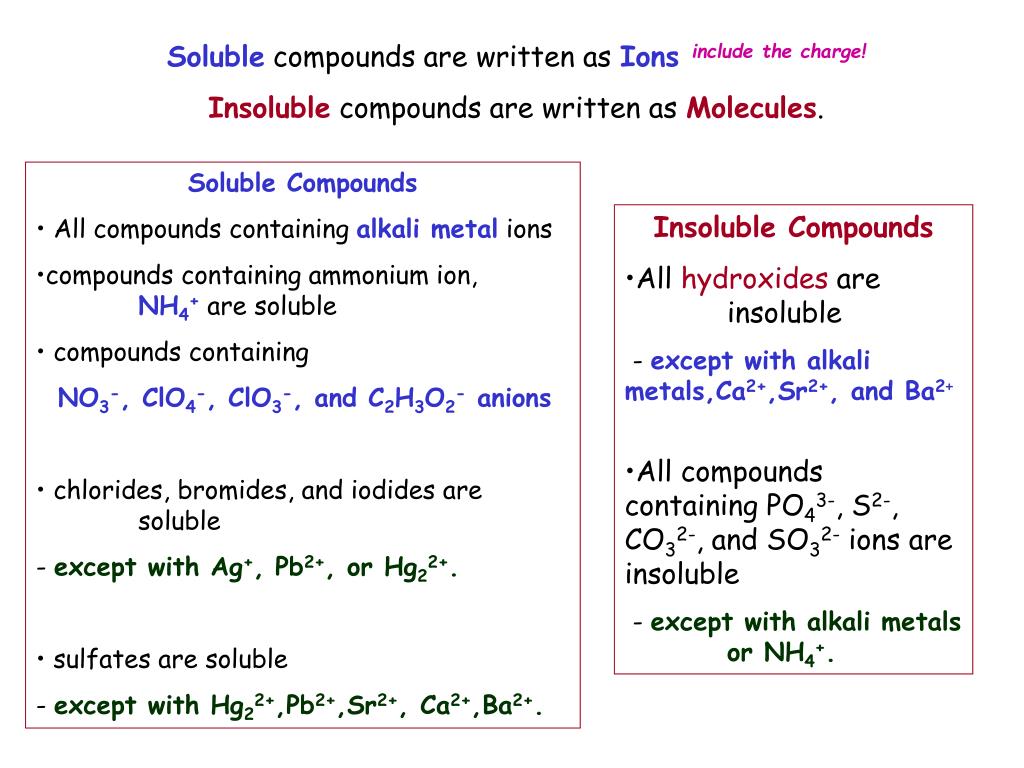
Why Are Ionic Compounds Soluble In Water Class 10 . Polar solvent h 2 o dissociates the ionic compound by interacting. The extent to which a substance may be dissolved. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Solubility of ionic compounds in water. The solubility of ionic compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Water is a polar solvent. Water molecules are polar because oxygen atoms are.
from www.slideserve.com
Polar solvent h 2 o dissociates the ionic compound by interacting. The solubility of ionic compounds. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. The extent to which a substance may be dissolved. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Water is a polar solvent. Water molecules are polar because oxygen atoms are. Solubility of ionic compounds in water. Predict solubilities of ionic compounds in water using the solubility rules.
PPT With enough water molecules, a soluble ionic compound is
Why Are Ionic Compounds Soluble In Water Class 10 Water molecules are polar because oxygen atoms are. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. The solubility of ionic compounds. Water molecules are polar because oxygen atoms are. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Water is a polar solvent. Polar solvent h 2 o dissociates the ionic compound by interacting. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Predict solubilities of ionic compounds in water using the solubility rules. Solubility of ionic compounds in water. The extent to which a substance may be dissolved.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is Why Are Ionic Compounds Soluble In Water Class 10 Predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance may be dissolved. Water is a polar solvent. Polar solvent h 2 o dissociates the ionic compound by interacting. Water molecules are polar because oxygen atoms are. Solubility of ionic compounds in water. When ionic compounds dissolve in water, the ions in the. Why Are Ionic Compounds Soluble In Water Class 10.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water Why Are Ionic Compounds Soluble In Water Class 10 The extent to which a substance may be dissolved. Solubility of ionic compounds in water. Polar solvent h 2 o dissociates the ionic compound by interacting. The solubility of ionic compounds. Water is a polar solvent. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Ionic. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Why Are Ionic Compounds Soluble In Water Class 10 The extent to which a substance may be dissolved. The solubility of ionic compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Water is a polar solvent. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. Why Are Ionic Compounds Soluble In Water Class 10.
From favpng.com
Ionic Compound Sodium Chloride Electrical Conductivity Water, PNG Why Are Ionic Compounds Soluble In Water Class 10 When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Solubility of ionic compounds in water. The extent to which a substance may. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free Why Are Ionic Compounds Soluble In Water Class 10 The extent to which a substance may be dissolved. Polar solvent h 2 o dissociates the ionic compound by interacting. Water molecules are polar because oxygen atoms are. Predict solubilities of ionic compounds in water using the solubility rules. Solubility of ionic compounds in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Why Are Ionic Compounds Soluble In Water Class 10.
From www.chegg.com
Solved 3. Why are some ionic compounds soluble in water and Why Are Ionic Compounds Soluble In Water Class 10 Solubility of ionic compounds in water. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing. Why Are Ionic Compounds Soluble In Water Class 10.
From study.com
Compound Solubility in Water Overview & Examples Lesson Why Are Ionic Compounds Soluble In Water Class 10 The extent to which a substance may be dissolved. The solubility of ionic compounds. Water is a polar solvent. Polar solvent h 2 o dissociates the ionic compound by interacting. Solubility of ionic compounds in water. Water molecules are polar because oxygen atoms are. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the. Why Are Ionic Compounds Soluble In Water Class 10.
From www.numerade.com
SOLVED Solubility Rulesfor some ionic compounds in water Soluble Ionic Why Are Ionic Compounds Soluble In Water Class 10 The extent to which a substance may be dissolved. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Water molecules are polar because oxygen atoms are. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water.. Why Are Ionic Compounds Soluble In Water Class 10.
From kunduz.com
[ANSWERED] Which of the following ionic compounds is soluble in water Why Are Ionic Compounds Soluble In Water Class 10 Solubility of ionic compounds in water. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Predict solubilities of ionic compounds in water. Why Are Ionic Compounds Soluble In Water Class 10.
From www.numerade.com
SOLVEDWhy are the ionic compounds soluble in water? Why Are Ionic Compounds Soluble In Water Class 10 When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. The solubility of ionic compounds. Solubility. Why Are Ionic Compounds Soluble In Water Class 10.
From www.studypool.com
SOLUTION Solubility of ionic compounds Studypool Why Are Ionic Compounds Soluble In Water Class 10 Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance may be dissolved. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation Why Are Ionic Compounds Soluble In Water Class 10 Predict solubilities of ionic compounds in water using the solubility rules. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Solubility of. Why Are Ionic Compounds Soluble In Water Class 10.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube Why Are Ionic Compounds Soluble In Water Class 10 The solubility of ionic compounds. Polar solvent h 2 o dissociates the ionic compound by interacting. Water molecules are polar because oxygen atoms are. Predict solubilities of ionic compounds in water using the solubility rules. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. The. Why Are Ionic Compounds Soluble In Water Class 10.
From www.chegg.com
Solved Are all ionic compounds soluble in water? Why Are Ionic Compounds Soluble In Water Class 10 Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Water molecules are polar because oxygen atoms are. Predict solubilities of ionic compounds in water using the solubility rules. The solubility of ionic compounds. Polar solvent h 2 o dissociates the ionic compound by interacting. Water. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT Bonding PowerPoint Presentation ID3050946 Why Are Ionic Compounds Soluble In Water Class 10 Water molecules are polar because oxygen atoms are. The solubility of ionic compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance may be dissolved. Solubility of ionic compounds. Why Are Ionic Compounds Soluble In Water Class 10.
From studymind.co.uk
Ionic Compound Properties (GCSE Chemistry) Study Mind Why Are Ionic Compounds Soluble In Water Class 10 Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. When ionic. Why Are Ionic Compounds Soluble In Water Class 10.
From www.msjchem.com
Structure 2.1 The ionic model MSJChem Tutorial videos for IB Chemistry Why Are Ionic Compounds Soluble In Water Class 10 Water molecules are polar because oxygen atoms are. Water is a polar solvent. Polar solvent h 2 o dissociates the ionic compound by interacting. Solubility of ionic compounds in water. Predict solubilities of ionic compounds in water using the solubility rules. The solubility of ionic compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse. Why Are Ionic Compounds Soluble In Water Class 10.
From www.numerade.com
SOLVED Text Solubility of Ionic Compounds Consult the solubility Why Are Ionic Compounds Soluble In Water Class 10 Water is a polar solvent. The solubility of ionic compounds. Solubility of ionic compounds in water. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in. Why Are Ionic Compounds Soluble In Water Class 10.
From www.chemistryspace.com
Solubility Rules for Ionic Compounds Why Are Ionic Compounds Soluble In Water Class 10 Water molecules are polar because oxygen atoms are. Water is a polar solvent. Polar solvent h 2 o dissociates the ionic compound by interacting. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Solubility of ionic compounds in water. The solubility of ionic compounds. The. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Why Are Ionic Compounds Soluble In Water Class 10 Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Solubility of ionic compounds in water. Polar solvent h 2 o dissociates the ionic compound by interacting. Water is a polar solvent. When ionic compounds dissolve in water, the ions in the solid separate and disperse. Why Are Ionic Compounds Soluble In Water Class 10.
From chemcollective.org
CHEM 1315 Lab 10 Conservation of Mass Why Are Ionic Compounds Soluble In Water Class 10 Polar solvent h 2 o dissociates the ionic compound by interacting. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. Why Are Ionic Compounds Soluble In Water Class 10.
From mbsutexas.web.fc2.com
Why do ionic compounds conduct electricity in water Why Are Ionic Compounds Soluble In Water Class 10 The solubility of ionic compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Water molecules are polar because oxygen atoms are. Water is a polar. Why Are Ionic Compounds Soluble In Water Class 10.
From bceweb.org
Solubility Of Compounds In Water Chart A Visual Reference of Charts Why Are Ionic Compounds Soluble In Water Class 10 Polar solvent h 2 o dissociates the ionic compound by interacting. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Solubility of ionic compounds in. Why Are Ionic Compounds Soluble In Water Class 10.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water Why Are Ionic Compounds Soluble In Water Class 10 Polar solvent h 2 o dissociates the ionic compound by interacting. Predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance may be dissolved. The solubility of ionic compounds. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the.. Why Are Ionic Compounds Soluble In Water Class 10.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Why Are Ionic Compounds Soluble In Water Class 10 When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Water is a polar solvent. Predict solubilities of ionic compounds in water using the solubility rules.. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Why Are Ionic Compounds Soluble In Water Class 10 Predict solubilities of ionic compounds in water using the solubility rules. Polar solvent h 2 o dissociates the ionic compound by interacting. Solubility of ionic compounds in water. Water is a polar solvent. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. The extent to which. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Why Are Ionic Compounds Soluble In Water Class 10 The extent to which a substance may be dissolved. Water molecules are polar because oxygen atoms are. The solubility of ionic compounds. Polar solvent h 2 o dissociates the ionic compound by interacting. Solubility of ionic compounds in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is Why Are Ionic Compounds Soluble In Water Class 10 When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Solubility of ionic compounds in water. Water molecules are polar because oxygen atoms are. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing. Why Are Ionic Compounds Soluble In Water Class 10.
From slideplayer.com
Solubility equilibrium Entropy & Free Energy ppt download Why Are Ionic Compounds Soluble In Water Class 10 The solubility of ionic compounds. Predict solubilities of ionic compounds in water using the solubility rules. Solubility of ionic compounds in water. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. The extent to which a substance may be dissolved. Ionic compounds are soluble in water because water is. Why Are Ionic Compounds Soluble In Water Class 10.
From www.docsity.com
Solubility General Chemistry Lecture Slides Docsity Why Are Ionic Compounds Soluble In Water Class 10 Water is a polar solvent. Water molecules are polar because oxygen atoms are. Polar solvent h 2 o dissociates the ionic compound by interacting. The solubility of ionic compounds. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Ionic compounds are soluble in water because water is a polar. Why Are Ionic Compounds Soluble In Water Class 10.
From www.youtube.com
why ionic compounds are soluble in water? ( A proper reason ) YouTube Why Are Ionic Compounds Soluble In Water Class 10 Water is a polar solvent. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Solubility of ionic compounds in water. The solubility of ionic compounds. When ionic compounds dissolve in water, the ions in the solid separate and. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID Why Are Ionic Compounds Soluble In Water Class 10 When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Predict solubilities of ionic compounds in water using the solubility rules. The solubility. Why Are Ionic Compounds Soluble In Water Class 10.
From www.sliderbase.com
Properties of Water Presentation Biology Why Are Ionic Compounds Soluble In Water Class 10 When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. The extent to which a substance may be dissolved. Water is a polar solvent. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I Why Are Ionic Compounds Soluble In Water Class 10 Predict solubilities of ionic compounds in water using the solubility rules. Water is a polar solvent. The solubility of ionic compounds. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Water molecules are polar because oxygen atoms are. When ionic compounds dissolve in water, the. Why Are Ionic Compounds Soluble In Water Class 10.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Why Are Ionic Compounds Soluble In Water Class 10 The extent to which a substance may be dissolved. Water molecules are polar because oxygen atoms are. Polar solvent h 2 o dissociates the ionic compound by interacting. Ionic compounds are soluble in water because water is a polar molecule that can hydrate the ions, breaking the ionic bonds and allowing the. Water is a polar solvent. When ionic compounds. Why Are Ionic Compounds Soluble In Water Class 10.