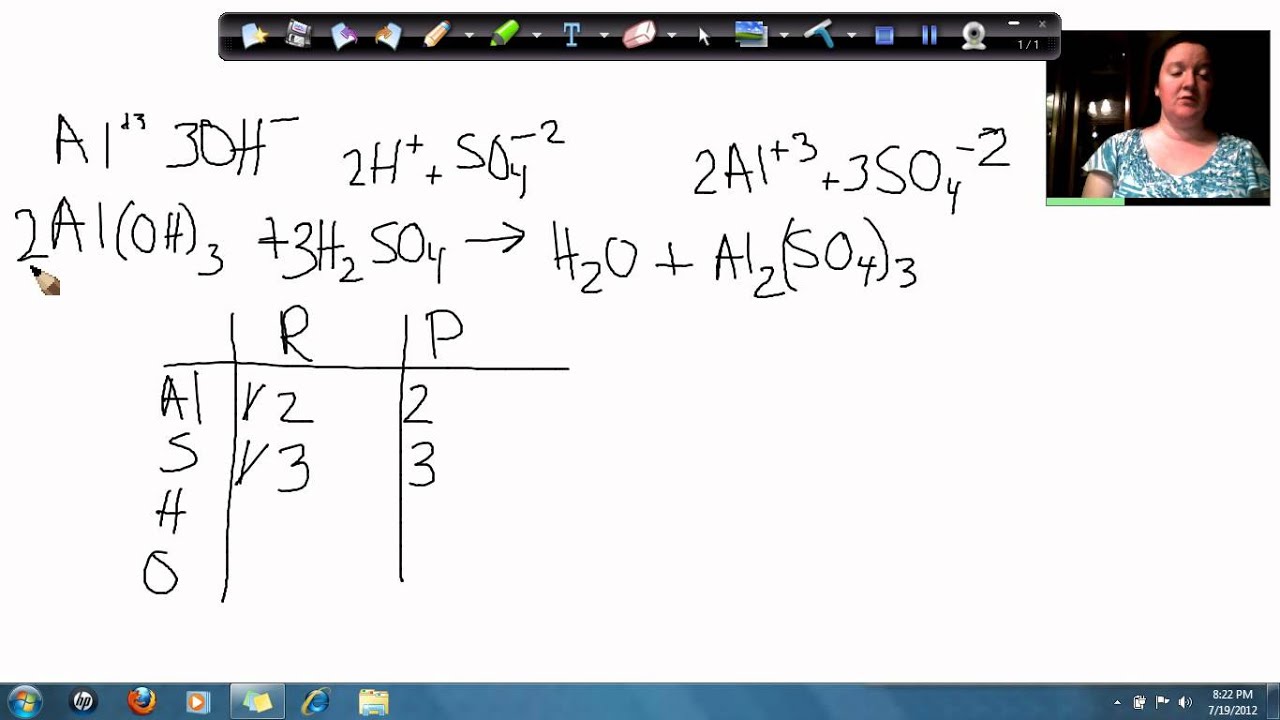
Aluminum Hydroxide And Sulfuric Acid . Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid as follows: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Aluminum hydroxide reacts with sulfuric acid as follows:
from www.youtube.com
Aluminum hydroxide reacts with sulfuric acid as follows: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows:
Aluminum Hydroxide And Sulfuric Acid Makes Water And Aluminum Sulfate
Aluminum Hydroxide And Sulfuric Acid 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: How many moles of al2(so4)3al2(so4)3 can form under these conditions?. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows:
From byjus.com
The balanced net ionic equation for the reaction of aluminium sulphate Aluminum Hydroxide And Sulfuric Acid 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid. Aluminum Hydroxide And Sulfuric Acid.
From testbook.com
Aluminium Sulfate Definition, Structure and its Applications. Aluminum Hydroxide And Sulfuric Acid 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Al2(so4)3 + hoh = al(oh)3. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVED 9 . Write a balanced neutralization reaction between the Aluminum Hydroxide And Sulfuric Acid Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o. Aluminum Hydroxide And Sulfuric Acid.
From www.saubhaya.com
Aluminium Hydroxide In Makeup Schädlich Saubhaya Makeup Aluminum Hydroxide And Sulfuric Acid 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid as follows: The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum sulfate + water = aluminum. Aluminum Hydroxide And Sulfuric Acid.
From www.youtube.com
Al2O3+H2SO4=Al2(SO4)3+H2O Balanced EquationAluminum oxide+Sulphuric Aluminum Hydroxide And Sulfuric Acid Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum hydroxide reacts with sulfuric acid as. Aluminum Hydroxide And Sulfuric Acid.
From science-direct-topics.blogspot.com
Science Direct Topics Aluminum Hydrochloric Acid Aluminum Hydroxide And Sulfuric Acid Aluminum hydroxide reacts with sulfuric acid as follows: How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). Aluminium reacts with sulfuric. Aluminum Hydroxide And Sulfuric Acid.
From questions.kunduz.com
Which of these shows the balanced reactio... Physical Chemistry Aluminum Hydroxide And Sulfuric Acid Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum hydroxide reacts with. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVED Aluminate ions combine with sulfuric acid to produce aluminum Aluminum Hydroxide And Sulfuric Acid Aluminum hydroxide reacts with sulfuric acid as follows: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). Aluminium reacts with sulfuric acid. Aluminum Hydroxide And Sulfuric Acid.
From www.youtube.com
Aluminum Hydroxide And Sulfuric Acid Makes Water And Aluminum Sulfate Aluminum Hydroxide And Sulfuric Acid Aluminum hydroxide reacts with sulfuric acid as follows: Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: How many moles of al2(so4)3al2(so4)3 can form under these conditions?. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. 2al (oh)3 (s)+3h2so4 (aq). Aluminum Hydroxide And Sulfuric Acid.
From www.slideshare.net
Reaction Of Metal Hydroxides With Acid Aluminum Hydroxide And Sulfuric Acid 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid as follows: Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). The first reaction that needs to be carried out is that. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVEDIf 3.00 g of aluminum hydroxide reacts with 1.00 g of sulfuric Aluminum Hydroxide And Sulfuric Acid Aluminum hydroxide reacts with sulfuric acid as follows: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminum hydroxide reacts with sulfuric acid as follows: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2. Aluminum Hydroxide And Sulfuric Acid.
From www.chegg.com
Solved Aluminum hydroxide reacts with sulfuric acid as Aluminum Hydroxide And Sulfuric Acid Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: Aluminum hydroxide reacts with sulfuric acid as follows: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Al2(so4)3 + hoh = al(oh)3. Aluminum Hydroxide And Sulfuric Acid.
From www.youtube.com
How to Write the Formula for Aluminum hydroxide YouTube Aluminum Hydroxide And Sulfuric Acid Aluminum hydroxide reacts with sulfuric acid as follows: The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Al2(so4)3 + hoh = al(oh)3 + h2so4 is. Aluminum Hydroxide And Sulfuric Acid.
From www.youtube.com
Metals Vs Sulfuric acid YouTube Aluminum Hydroxide And Sulfuric Acid 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: How many moles of al2(so4)3al2(so4)3 can form under. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVEDand net ionic equation for the following eactions Write Aluminum Hydroxide And Sulfuric Acid Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh:. Aluminum Hydroxide And Sulfuric Acid.
From www.chegg.com
Solved What would be the proper mechanism of the reaction of Aluminum Hydroxide And Sulfuric Acid The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum sulfate + water = aluminum hydroxide. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVED Chemical equation for the reaction of aluminium hydroxide and Aluminum Hydroxide And Sulfuric Acid 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum hydroxide reacts with sulfuric acid as follows: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid as follows: How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminum sulfate + water =. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVED a) Write the balanced chemical reaction for the following (2 Aluminum Hydroxide And Sulfuric Acid Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). How many moles of al2(so4)3al2(so4)3 can form. Aluminum Hydroxide And Sulfuric Acid.
From www.youtube.com
How to Balance Al2O3 + H2SO4 = Al2(SO4)3 + H2O (Aluminum oxide Aluminum Hydroxide And Sulfuric Acid How many moles of al2(so4)3al2(so4)3 can form under these conditions?. 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. 2 al1oh231s2 + 3. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVED 3 Limiting Reactant 1. Aluminum hydroxide reacts with sulfuric Aluminum Hydroxide And Sulfuric Acid Aluminum hydroxide reacts with sulfuric acid as follows: Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVED Aluminum hydroxide reacts with sulfuric acid as follows 2 Al Aluminum Hydroxide And Sulfuric Acid Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh:. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVED Sulfuric acid reacts with aluminum hydroxide by double Aluminum Hydroxide And Sulfuric Acid Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Aluminum hydroxide reacts with sulfuric acid as follows: 2. Aluminum Hydroxide And Sulfuric Acid.
From www.doubtnut.com
(a) Aluminium hydroxide reacts with sulphuric acid to from aluminium s Aluminum Hydroxide And Sulfuric Acid The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis).. Aluminum Hydroxide And Sulfuric Acid.
From www.youtube.com
(a) Aluminium hydroxide reacts with sulphuric acid to form aluminium Aluminum Hydroxide And Sulfuric Acid The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). How many moles of al2(so4)3al2(so4)3 can form under these conditions?. 2al. Aluminum Hydroxide And Sulfuric Acid.
From askfilo.com
Aluminium reacts with sulfuric acid to form aluminium sulfate and hydroge.. Aluminum Hydroxide And Sulfuric Acid 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6. Aluminum Hydroxide And Sulfuric Acid.
From brainly.com
The following reaction shows the products when sulfuric acid and Aluminum Hydroxide And Sulfuric Acid 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Aluminum. Aluminum Hydroxide And Sulfuric Acid.
From w20.b2m.cz
Ai Oh 3 H2so4 Ai2 So4 3 H2o EDUCA Aluminum Hydroxide And Sulfuric Acid How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen. Aluminum Hydroxide And Sulfuric Acid.
From klazoofqu.blob.core.windows.net
Zinc Hydroxide And Sulfuric Acid at Mamie Hunter blog Aluminum Hydroxide And Sulfuric Acid Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,.. Aluminum Hydroxide And Sulfuric Acid.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Reaction Of Aluminium Oxide With Sulphuric Acid Aluminum Hydroxide And Sulfuric Acid 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. How many moles of al2(so4)3al2(so4)3 can form under. Aluminum Hydroxide And Sulfuric Acid.
From askfilo.com
24. (a) Aluminium hydroxide reacts with sulphuric acid to form aluminium Aluminum Hydroxide And Sulfuric Acid Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid as follows: Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 +. Aluminum Hydroxide And Sulfuric Acid.
From www.numerade.com
SOLVED The following is an acidbase reaction between sulfuric acid Aluminum Hydroxide And Sulfuric Acid Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum hydroxide reacts with sulfuric acid as follows: How many moles of al2(so4)3al2(so4)3 can form under these conditions?. Aluminum hydroxide reacts with sulfuric acid as follows: The first reaction that needs to be carried out is that of aluminum and potassium. Aluminum Hydroxide And Sulfuric Acid.
From www.youtube.com
Sulfuric acid plus aluminum hydroxide YouTube Aluminum Hydroxide And Sulfuric Acid Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Aluminum hydroxide reacts with sulfuric acid as follows: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of. Aluminum Hydroxide And Sulfuric Acid.
From www.youtube.com
(a) Aluminium hydroxide reacts with sulphuric acid to form aluminiu Aluminum Hydroxide And Sulfuric Acid Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. The first reaction that needs to be carried out is that of aluminum and potassium hydroxide, koh: Aluminum hydroxide reacts with sulfuric acid as follows: 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. Aluminium reacts with sulfuric acid (h2so4) to produce. Aluminum Hydroxide And Sulfuric Acid.
From questions.kunduz.com
Question no. A (22 pts) The following is Physical Chemistry Aluminum Hydroxide And Sulfuric Acid Aluminum hydroxide reacts with sulfuric acid as follows: 2al (oh)3 (s)+3h2so4 (aq) al2 (so4)3 (aq)+6h2o (l) if 0.357 mol aluminum hydroxide reacts with 0.468 mol of sulfuric acid,. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminium reacts with sulfuric acid (h2so4) to produce aluminium sulfate al2 (so4)3 and hydrogen (h2). Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum. Aluminum Hydroxide And Sulfuric Acid.
From questions.kunduz.com
The following is an acidbase reaction bet... Organic Chemistry Aluminum Hydroxide And Sulfuric Acid Aluminum sulfate + water = aluminum hydroxide + sulfuric acid. Aluminum hydroxide reacts with sulfuric acid as follows: Aluminum hydroxide reacts with sulfuric acid as follows: Al2(so4)3 + hoh = al(oh)3 + h2so4 is a double displacement (metathesis). How many moles of al2(so4)3al2(so4)3 can form under these conditions?. 2 al1oh231s2 + 3 h2so41aq2¡al21so4231aq2 + 6 h2o1l2 how. The first reaction. Aluminum Hydroxide And Sulfuric Acid.