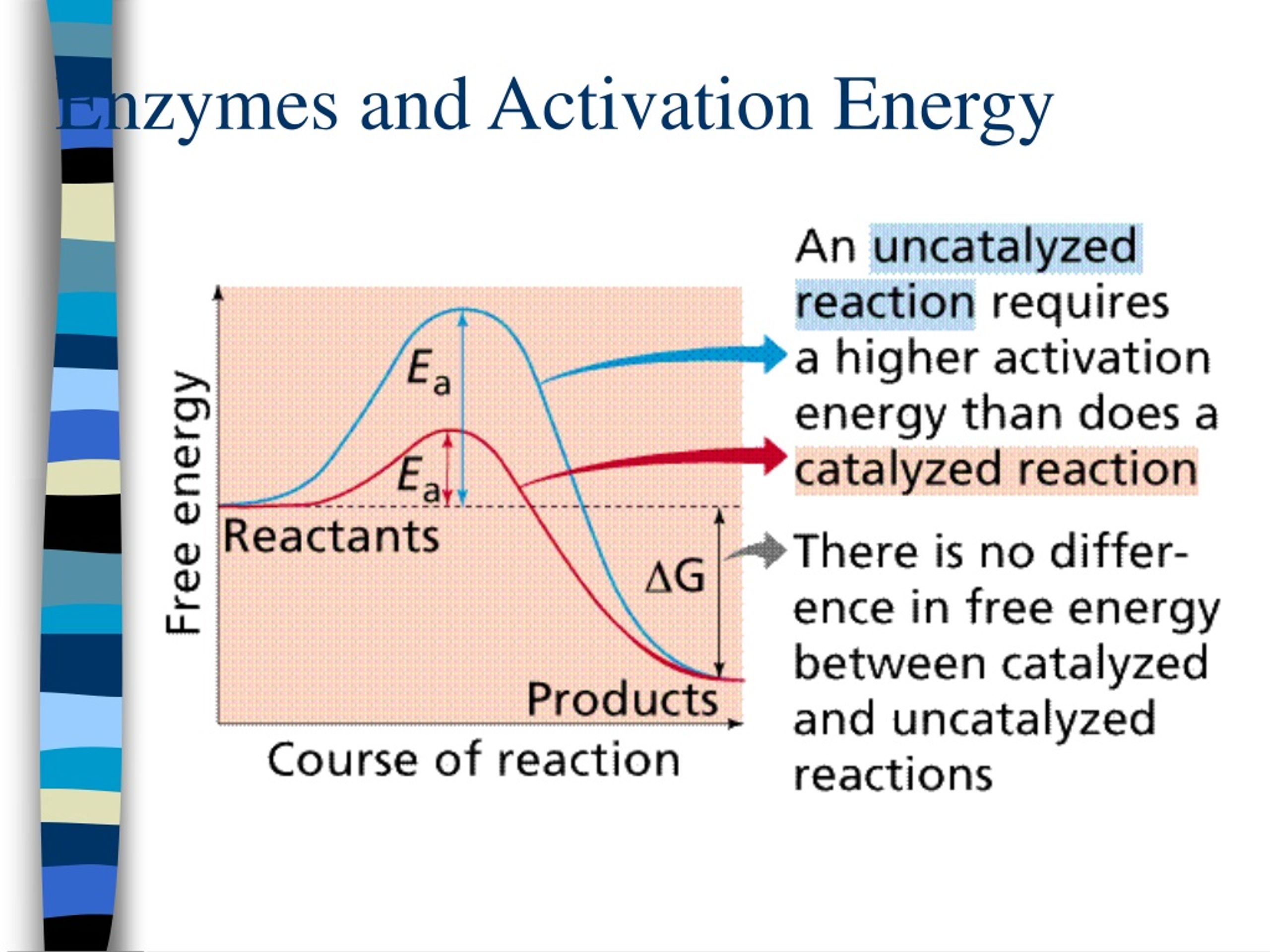
Do Catalysts Decrease Activation Energy . Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Instead they provide a new mechanism for a reaction to occur which. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. When a catalyst is used at 300 k, the rate increases one hundred fold. The activation energy of a reaction is 19.0 kj/mol. A catalyst can open a new reaction pathway with lower activation energy. The position of activation energy can be. This does not change the frequency of collisions. The key importance of activation energy. However, it does increase the frequency of successful collisions because. It does it by providing a partial bond which stabilises the transition. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts.
from loepqgkec.blob.core.windows.net
For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. The activation energy of a reaction is 19.0 kj/mol. The key importance of activation energy. Instead they provide a new mechanism for a reaction to occur which. A catalyst can open a new reaction pathway with lower activation energy. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. The position of activation energy can be. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. However, it does increase the frequency of successful collisions because. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction.
Do Enzymes Regulate Chemical Reactions at Bethany Pasco blog
Do Catalysts Decrease Activation Energy A catalyst can open a new reaction pathway with lower activation energy. This does not change the frequency of collisions. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The key importance of activation energy. The position of activation energy can be. When a catalyst is used at 300 k, the rate increases one hundred fold. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. The activation energy of a reaction is 19.0 kj/mol. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. A catalyst can open a new reaction pathway with lower activation energy. It does it by providing a partial bond which stabilises the transition. Instead they provide a new mechanism for a reaction to occur which. However, it does increase the frequency of successful collisions because.
From 2012books.lardbucket.org
Catalysis Do Catalysts Decrease Activation Energy Instead they provide a new mechanism for a reaction to occur which. The key importance of activation energy. When a catalyst is used at 300 k, the rate increases one hundred fold. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. The activation energy of a reaction is 19.0 kj/mol. This does. Do Catalysts Decrease Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Do Catalysts Decrease Activation Energy Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Instead they provide a new mechanism for a reaction to occur which. It does it by providing a partial bond which stabilises the transition. The position of activation energy can be. A catalyst can open a new reaction pathway with lower activation energy.. Do Catalysts Decrease Activation Energy.
From studyonline.blog
What Is Activation Energy? Definition and Examples Do Catalysts Decrease Activation Energy Instead they provide a new mechanism for a reaction to occur which. However, it does increase the frequency of successful collisions because. A catalyst can open a new reaction pathway with lower activation energy. The activation energy of a reaction is 19.0 kj/mol. The key importance of activation energy. When a catalyst is used at 300 k, the rate increases. Do Catalysts Decrease Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Do Catalysts Decrease Activation Energy The key importance of activation energy. This does not change the frequency of collisions. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Instead they provide a new mechanism for a reaction to occur which. The activation energy of a reaction is 19.0 kj/mol. A catalyst can. Do Catalysts Decrease Activation Energy.
From schoolbag.info
CATALYSTS AND ENERGY DIAGRAMS Chemical Reactions, Energy Changes, and Do Catalysts Decrease Activation Energy However, it does increase the frequency of successful collisions because. The position of activation energy can be. It does it by providing a partial bond which stabilises the transition. This does not change the frequency of collisions. Instead they provide a new mechanism for a reaction to occur which. Catalysts are defined as substances that participate in a chemical reaction. Do Catalysts Decrease Activation Energy.
From physics.stackexchange.com
material science What properties make a good catalyst? Physics Do Catalysts Decrease Activation Energy This does not change the frequency of collisions. Instead they provide a new mechanism for a reaction to occur which. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. However, it does increase the frequency of successful collisions because. Catalysts are defined as substances that participate in. Do Catalysts Decrease Activation Energy.
From loepqgkec.blob.core.windows.net
Do Enzymes Regulate Chemical Reactions at Bethany Pasco blog Do Catalysts Decrease Activation Energy However, it does increase the frequency of successful collisions because. The activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at 300 k, the rate increases one hundred fold. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The position of activation energy. Do Catalysts Decrease Activation Energy.
From professional.patrickneyman.com
Activation Energy Equation Do Catalysts Decrease Activation Energy The key importance of activation energy. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. It does it by providing a partial bond which stabilises the transition.. Do Catalysts Decrease Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Do Catalysts Decrease Activation Energy The position of activation energy can be. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. A catalyst can open a new reaction pathway with lower activation energy. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called. Do Catalysts Decrease Activation Energy.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Do Catalysts Decrease Activation Energy The position of activation energy can be. The key importance of activation energy. Instead they provide a new mechanism for a reaction to occur which. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Collisions only result in a reaction if the particles collide with a certain. Do Catalysts Decrease Activation Energy.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Do Catalysts Decrease Activation Energy However, it does increase the frequency of successful collisions because. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. It does it by providing a partial bond which stabilises the transition. Catalysts are defined as substances that participate in a chemical reaction but are not changed or. Do Catalysts Decrease Activation Energy.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Do Catalysts Decrease Activation Energy The activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at 300 k, the rate increases one hundred fold. The position of activation energy can be. However, it does increase the frequency of successful collisions because. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts.. Do Catalysts Decrease Activation Energy.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Do Catalysts Decrease Activation Energy Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. The key importance of activation energy. When a catalyst is used at 300 k, the rate increases one hundred fold. However, it does increase the frequency of successful collisions because. It does it by providing a partial bond which stabilises the transition. This. Do Catalysts Decrease Activation Energy.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free Do Catalysts Decrease Activation Energy Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. The key importance of activation energy. When a catalyst is used at 300 k, the rate increases one hundred fold. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Collisions. Do Catalysts Decrease Activation Energy.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Do Catalysts Decrease Activation Energy When a catalyst is used at 300 k, the rate increases one hundred fold. The activation energy of a reaction is 19.0 kj/mol. The position of activation energy can be. This does not change the frequency of collisions. It does it by providing a partial bond which stabilises the transition. Instead they provide a new mechanism for a reaction to. Do Catalysts Decrease Activation Energy.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Do Catalysts Decrease Activation Energy This does not change the frequency of collisions. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The activation energy of a reaction is 19.0 kj/mol. A catalyst can open a new reaction pathway with lower activation energy. However, it does increase the frequency of successful collisions. Do Catalysts Decrease Activation Energy.
From byjus.com
How does a catalyst increase the rate of a reaction? Do Catalysts Decrease Activation Energy However, it does increase the frequency of successful collisions because. It does it by providing a partial bond which stabilises the transition. A catalyst can open a new reaction pathway with lower activation energy. The key importance of activation energy. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for. Do Catalysts Decrease Activation Energy.
From loepqgkec.blob.core.windows.net
Do Enzymes Regulate Chemical Reactions at Bethany Pasco blog Do Catalysts Decrease Activation Energy It does it by providing a partial bond which stabilises the transition. This does not change the frequency of collisions. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. However, it does increase the frequency of successful collisions because. For cellular reactions to occur fast enough over. Do Catalysts Decrease Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Do Catalysts Decrease Activation Energy The key importance of activation energy. It does it by providing a partial bond which stabilises the transition. The activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at 300 k, the rate increases one hundred fold. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy. Do Catalysts Decrease Activation Energy.
From cheminfo.uz
Kataliz va katalizator haqida ChemInfo.uz Do Catalysts Decrease Activation Energy The position of activation energy can be. This does not change the frequency of collisions. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Instead they provide a new mechanism for a reaction to occur which. However, it does increase the frequency of successful collisions because. Collisions. Do Catalysts Decrease Activation Energy.
From exodjtdpy.blob.core.windows.net
Enzymes Raise The Activation Energy Of A Reaction at Sean Ferguson blog Do Catalysts Decrease Activation Energy It does it by providing a partial bond which stabilises the transition. When a catalyst is used at 300 k, the rate increases one hundred fold. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The key importance of activation energy. For cellular reactions to occur fast. Do Catalysts Decrease Activation Energy.
From www.slideserve.com
PPT Basic Enzymology PowerPoint Presentation ID364307 Do Catalysts Decrease Activation Energy For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. The activation energy of a reaction is 19.0 kj/mol. However, it does increase the frequency of successful collisions because. The key importance of activation energy. Instead they provide a new mechanism for a reaction to occur which. It does it. Do Catalysts Decrease Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Do Catalysts Decrease Activation Energy It does it by providing a partial bond which stabilises the transition. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. However, it does increase the frequency of successful collisions because. The activation energy of a reaction is 19.0 kj/mol. Instead they provide a new mechanism for a reaction. Do Catalysts Decrease Activation Energy.
From www.slideserve.com
PPT EnZyMeS PowerPoint Presentation, free download ID2065739 Do Catalysts Decrease Activation Energy However, it does increase the frequency of successful collisions because. This does not change the frequency of collisions. When a catalyst is used at 300 k, the rate increases one hundred fold. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Collisions only result in a reaction. Do Catalysts Decrease Activation Energy.
From www.sliderbase.com
Catalysis Presentation Chemistry Do Catalysts Decrease Activation Energy This does not change the frequency of collisions. A catalyst can open a new reaction pathway with lower activation energy. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. The position of. Do Catalysts Decrease Activation Energy.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Do Catalysts Decrease Activation Energy Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. However, it does increase the frequency of successful collisions because. The key importance of activation energy. This does not change the frequency of collisions. The position of activation energy can be. Instead they provide a new mechanism for a reaction to occur which.. Do Catalysts Decrease Activation Energy.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Do Catalysts Decrease Activation Energy A catalyst can open a new reaction pathway with lower activation energy. The activation energy of a reaction is 19.0 kj/mol. Instead they provide a new mechanism for a reaction to occur which. This does not change the frequency of collisions. The key importance of activation energy. It does it by providing a partial bond which stabilises the transition. Collisions. Do Catalysts Decrease Activation Energy.
From www.chegg.com
Solved Which of the following statements about catalysts are Do Catalysts Decrease Activation Energy A catalyst can open a new reaction pathway with lower activation energy. When a catalyst is used at 300 k, the rate increases one hundred fold. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. However, it does increase the frequency of successful collisions because. This does. Do Catalysts Decrease Activation Energy.
From exohcytva.blob.core.windows.net
How Do Enzymes Lower Activation Energy Quizlet at Su Happel blog Do Catalysts Decrease Activation Energy The key importance of activation energy. The activation energy of a reaction is 19.0 kj/mol. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. This does not change the frequency of collisions. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for. Do Catalysts Decrease Activation Energy.
From infinitylearn.com
A catalyst increases the rate of reaction by Sri Chaitanya Infinity Do Catalysts Decrease Activation Energy It does it by providing a partial bond which stabilises the transition. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The position of activation energy can be. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called. Do Catalysts Decrease Activation Energy.
From socratic.org
Why does a catalyst cause a reaction to speed up? Socratic Do Catalysts Decrease Activation Energy The activation energy of a reaction is 19.0 kj/mol. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. The key importance of activation energy. The position of. Do Catalysts Decrease Activation Energy.
From study.com
Effect of Catalysts on Rates of Reaction Video & Lesson Transcript Do Catalysts Decrease Activation Energy For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. A catalyst can open a new reaction pathway with lower activation energy. It does it by providing a. Do Catalysts Decrease Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Do Catalysts Decrease Activation Energy The activation energy of a reaction is 19.0 kj/mol. A catalyst can open a new reaction pathway with lower activation energy. The key importance of activation energy. The position of activation energy can be. For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules called catalysts. When a catalyst is used at. Do Catalysts Decrease Activation Energy.
From photoideass.blogspot.com
How Do Heterogeneous Catalysts Work Photos Idea Do Catalysts Decrease Activation Energy The activation energy of a reaction is 19.0 kj/mol. This does not change the frequency of collisions. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. It does it by. Do Catalysts Decrease Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Do Catalysts Decrease Activation Energy It does it by providing a partial bond which stabilises the transition. When a catalyst is used at 300 k, the rate increases one hundred fold. Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. Catalysts are defined as substances that participate in a chemical reaction but. Do Catalysts Decrease Activation Energy.