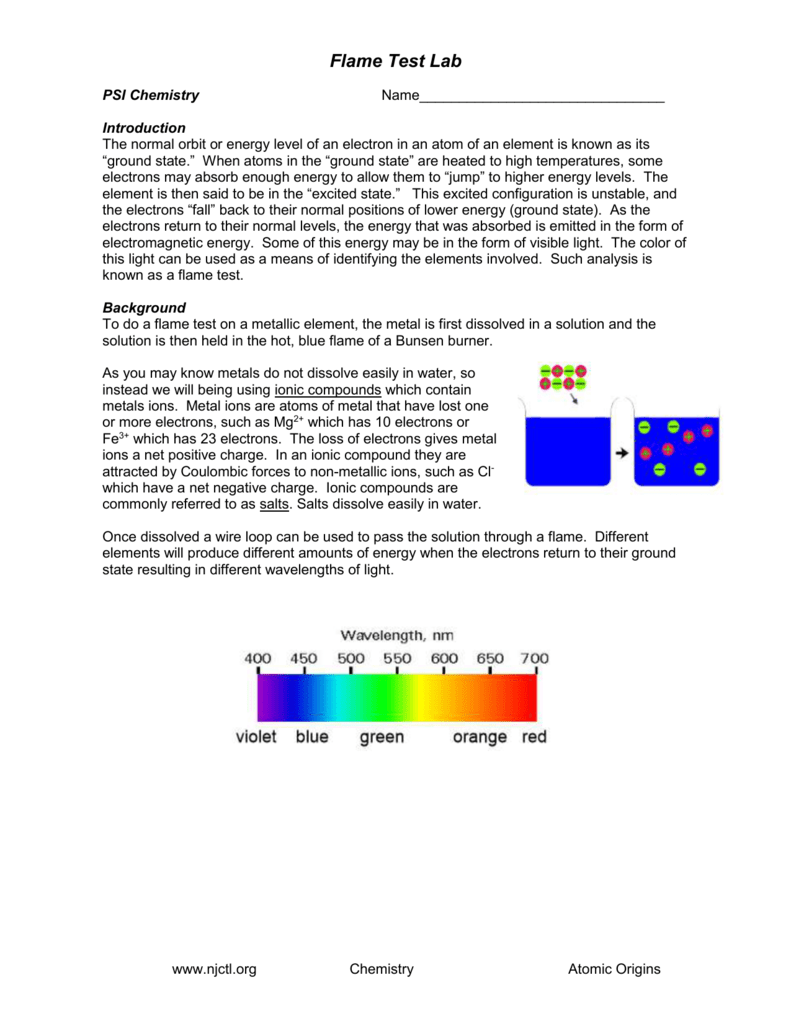
Flame Test Lab Handout . Then determine the identity of four unknown powders. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. What do the table salt, street lamp, and fireworks have in common? Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. They all contain sodium, which gives off a unique flame when heated. Adjust the gas and oxygen flow to produce. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders.
from studylib.net
Adjust the gas and oxygen flow to produce. What do the table salt, street lamp, and fireworks have in common? Then determine the identity of four unknown powders. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. They all contain sodium, which gives off a unique flame when heated. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to.
Flame Test Lab
Flame Test Lab Handout The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Then determine the identity of four unknown powders. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. What do the table salt, street lamp, and fireworks have in common? To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. Adjust the gas and oxygen flow to produce. They all contain sodium, which gives off a unique flame when heated.
From myans.bhantedhammika.net
Flame Test Lab Pdf Flame Test Lab Handout The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. Adjust the gas and oxygen flow to produce. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. Use a ame test and a spectroscope. Flame Test Lab Handout.
From www.studocu.com
L3 Flame Tests Lab 3 Work Lab Activity Flame Tests Problem Do all Flame Test Lab Handout Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the. Flame Test Lab Handout.
From ctdmhe.blogspot.com
Megan's Chemistry Lab Blog Lab 7 Flame Test Lab Flame Test Lab Handout To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. They all contain sodium, which gives off a unique flame when heated. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. Then determine the identity of four unknown powders. The purpose of the. Flame Test Lab Handout.
From www.scribd.com
Lab Flame Test Handout Chem PDF Emission Spectrum Scientific Flame Test Lab Handout The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. Then determine the identity of four unknown powders. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of. Flame Test Lab Handout.
From www.studocu.com
Flame Test Lab Report Prof. McLoud Flame Test Lab Report Flame Test Lab Handout The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. Identify the identity of the unknown using, not only the flame color, but. Flame Test Lab Handout.
From myans.bhantedhammika.net
Virtual Flame Test Lab Answer Key Flame Test Lab Handout Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. They all contain sodium, which gives off a unique flame when heated. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. The flame test is used. Flame Test Lab Handout.
From www.youtube.com
Flame Test Lab YouTube Flame Test Lab Handout They all contain sodium, which gives off a unique flame when heated. Then determine the identity of four unknown powders. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame. Flame Test Lab Handout.
From studylib.net
Flame Tests Lab Flame Test Lab Handout The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. They all contain sodium, which gives off a unique flame when heated. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Use. Flame Test Lab Handout.
From studylib.net
FLAME TEST LAB PROCEDURE Flame Test Lab Handout To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. Adjust the gas and oxygen flow to produce. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. The purpose of the flame. Flame Test Lab Handout.
From studylib.net
Flame Test lab handout Flame Test Lab Handout Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. Adjust the gas and oxygen flow to produce. Then determine the identity of four unknown powders. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. What. Flame Test Lab Handout.
From www.docsity.com
Flame test lab part 2 Study Guides, Projects, Research Chemistry Flame Test Lab Handout Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. What do the table salt, street lamp, and fireworks have in common? Use a ame. Flame Test Lab Handout.
From www.studocu.com
CHEM Flame LAB 2 chem lab Flame Test Lab Experimental Report Sheet Flame Test Lab Handout They all contain sodium, which gives off a unique flame when heated. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Use a ame test and a spectroscope to determine the. Flame Test Lab Handout.
From slidesharetrick.blogspot.com
Chemistry Flame Test Lab Answers slidesharetrick Flame Test Lab Handout The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. What do the table salt, street lamp, and fireworks have in common? Adjust the gas and oxygen flow to produce. Then determine the identity of four unknown powders. Identify the identity of the unknown. Flame Test Lab Handout.
From studylib.net
Flame Test Lab Flame Test Lab Handout They all contain sodium, which gives off a unique flame when heated. Adjust the gas and oxygen flow to produce. Then determine the identity of four unknown powders. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Use a ame test and a spectroscope to determine the emission line spectrum. Flame Test Lab Handout.
From www.youtube.com
Flame Test Lab YouTube Flame Test Lab Handout Then determine the identity of four unknown powders. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. What do the table salt,. Flame Test Lab Handout.
From studylib.net
Flame Test Lab Work Flame Test Lab Handout The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. What do the table salt, street lamp, and fireworks have in common? They all contain sodium, which gives off a unique flame when heated. Then determine the identity of four unknown powders. Use a. Flame Test Lab Handout.
From www.animalia-life.club
Flame Test Lab Results Flame Test Lab Handout What do the table salt, street lamp, and fireworks have in common? Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Then determine the identity of four unknown powders. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in. Flame Test Lab Handout.
From www.animalia-life.club
Flame Test Lab Results Flame Test Lab Handout What do the table salt, street lamp, and fireworks have in common? Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. They all contain sodium, which gives off a unique flame when heated. To observe and measure the atomic spectra of several elements and then use those measurements to make. Flame Test Lab Handout.
From www.animalia-life.club
Flame Test Lab Results Flame Test Lab Handout The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. What do the table salt, street. Flame Test Lab Handout.
From www.studocu.com
Copy of Flame Test Virtual Lab Flame Test Virtual Lab Document Flame Test Lab Handout Then determine the identity of four unknown powders. What do the table salt, street lamp, and fireworks have in common? They all contain sodium, which gives off a unique flame when heated. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. To observe and measure the atomic spectra of several elements and. Flame Test Lab Handout.
From www.docsity.com
Flame Test Lab Worksheet Activity with Answers Key Exercises Flame Test Lab Handout Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. What do the table salt, street lamp, and fireworks have in common? To observe and measure the. Flame Test Lab Handout.
From studylib.net
5.Flame Test Lab Flame Test Lab Handout To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. What do the table salt, street lamp, and fireworks have in common? They all contain sodium, which gives off a unique flame when heated. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders.. Flame Test Lab Handout.
From www.chegg.com
Solved Flame Tests Lab Data Table A. Known Metal cations Flame Test Lab Handout Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. Adjust the gas and oxygen flow to produce. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. The flame test is used to visually determine the identity of an unknown metal ion based. Flame Test Lab Handout.
From studylib.net
Flame Test Exploration Flame Test Lab Handout Adjust the gas and oxygen flow to produce. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. Then determine the identity of four unknown. Flame Test Lab Handout.
From kelseyreavy.com
Why You Need to do the Flame Test Lab in Your High School Chemistry Flame Test Lab Handout Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Then determine the identity of four unknown powders. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. Use a ame test and a spectroscope to determine the emission line spectrum of. Flame Test Lab Handout.
From studylib.net
Flame Test Lab Flame Test Lab Handout Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Adjust the gas and oxygen flow to produce. They all contain sodium, which gives off a unique flame when heated. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic. Flame Test Lab Handout.
From www.chegg.com
Solved Flame Test Lab Introduction In this activity, you Flame Test Lab Handout Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. The flame test is used. Flame Test Lab Handout.
From www.animalia-life.club
Flame Test Lab Results Flame Test Lab Handout Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. The purpose of the flame. Flame Test Lab Handout.
From www.animalia-life.club
Flame Test Lab Results Flame Test Lab Handout They all contain sodium, which gives off a unique flame when heated. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. Then determine the identity of four unknown powders. Adjust the gas and oxygen flow to produce. The purpose of the flame test lab is to observe the characteristic colors. Flame Test Lab Handout.
From sciencelessonsthatrock.com
Chemistry Flame Test Lab Science Lessons That Rock Flame Test Lab Handout They all contain sodium, which gives off a unique flame when heated. Use a ame test and a spectroscope to determine the emission line spectrum of six dierent powders. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then to. To observe and measure the. Flame Test Lab Handout.
From ctdatmanshah.blogspot.com
Lab 11 Flame Test Lab Flame Test Lab Handout Adjust the gas and oxygen flow to produce. Then determine the identity of four unknown powders. Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. The purpose of the flame test lab is to observe the characteristic colors produced by certain metallic ions when vaporized in a flame and then. Flame Test Lab Handout.
From www.studocu.com
Flame Test Lab Report Chemistry for Science Majors Claudia Flame Test Lab Handout Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. They all contain sodium, which gives off a unique flame when heated. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. The. Flame Test Lab Handout.
From www.studocu.com
Flames+Test+Lab+Worksheet Name Date Flame Test Lab Experimental Flame Test Lab Handout Then determine the identity of four unknown powders. They all contain sodium, which gives off a unique flame when heated. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. Use a ame test and a spectroscope to determine the emission line. Flame Test Lab Handout.
From studylib.net
Flame Test Lab Flame Test Lab Handout Identify the identity of the unknown using, not only the flame color, but also the spectral lines and wavelengths. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. What do the table salt, street lamp, and fireworks have in common? Use a ame test and a spectroscope to determine the. Flame Test Lab Handout.
From athensmutualaid.net
Virtual Flame Test Lab Answer Key › Athens Mutual Student Corner Flame Test Lab Handout Adjust the gas and oxygen flow to produce. The flame test is used to visually determine the identity of an unknown metal ion based on the color the metallic salt turns the flame of a bunsen. To observe and measure the atomic spectra of several elements and then use those measurements to make conclusions about. Use a ame test and. Flame Test Lab Handout.