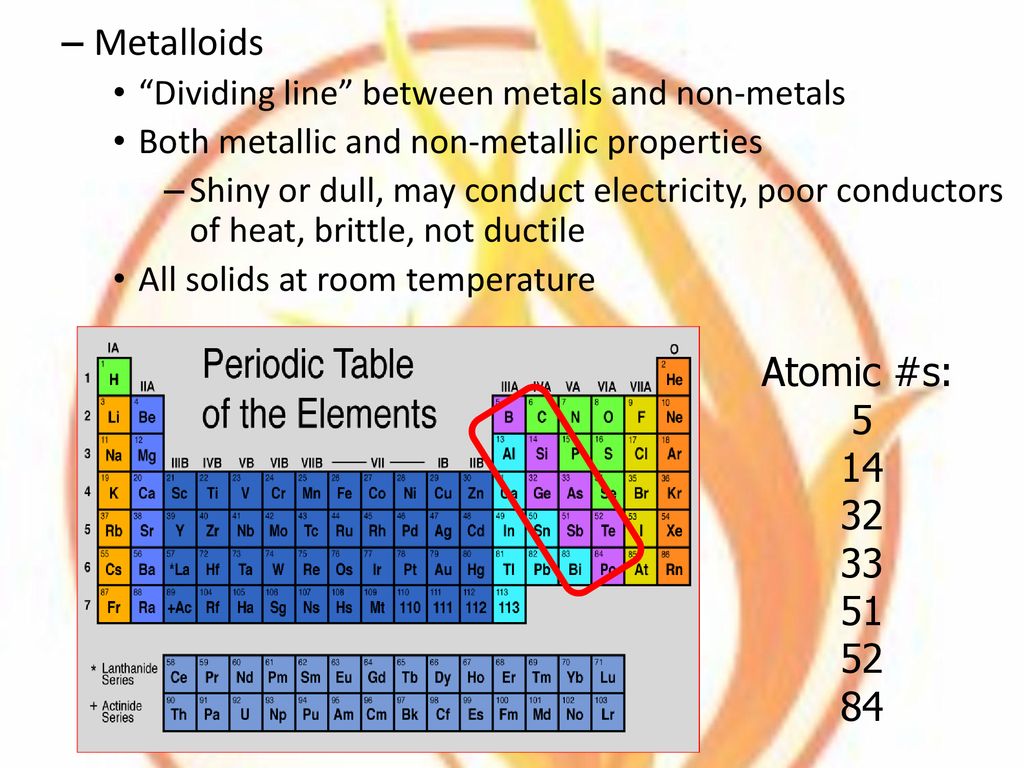
Are Metalloids Dull And Brittle . Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Location on the periodic table. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids or semimetals possess some properties of metals and some of nonmetals. Silicon is a metalloid because it has luster, but is brittle. Silicon for example appears lustrous, but is. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Properties of metalloids or semimetals. Metalloids typically have several forms or. Metalloids have semiconductor properties and form amphoteric oxides.
from slideplayer.com
Properties of metalloids or semimetals. Metalloids have semiconductor properties and form amphoteric oxides. Silicon for example appears lustrous, but is. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metalloids typically have several forms or. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Location on the periodic table. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Silicon is a metalloid because it has luster, but is brittle. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties.
Topic 3 Elements & the Periodic Table ppt download
Are Metalloids Dull And Brittle Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Properties of metalloids or semimetals. Silicon is a metalloid because it has luster, but is brittle. Metalloids or semimetals possess some properties of metals and some of nonmetals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Location on the periodic table. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Silicon for example appears lustrous, but is. Metalloids typically have several forms or. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids have semiconductor properties and form amphoteric oxides.
From slideplayer.com
Chapter 4 Atoms and Elements ppt download Are Metalloids Dull And Brittle Location on the periodic table. Silicon for example appears lustrous, but is. Metalloids have semiconductor properties and form amphoteric oxides. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Properties of metalloids or semimetals. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metals tend. Are Metalloids Dull And Brittle.
From slideplayer.com
The Periodic Table Chapter ppt download Are Metalloids Dull And Brittle Silicon is a metalloid because it has luster, but is brittle. Metalloids have semiconductor properties and form amphoteric oxides. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Location on the periodic table. Silicon for example appears lustrous, but is. Metalloids typically have several forms or. Metals tend. Are Metalloids Dull And Brittle.
From www.slideserve.com
PPT Chapter 6 Periodic Table PowerPoint Presentation, free download Are Metalloids Dull And Brittle Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids have semiconductor properties and form amphoteric oxides. Properties of metalloids or semimetals. Location on the periodic table. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they. Are Metalloids Dull And Brittle.
From www.slideserve.com
PPT Chapter 3 Atoms and Elements PowerPoint Presentation, free Are Metalloids Dull And Brittle Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they. Are Metalloids Dull And Brittle.
From www.slideserve.com
PPT The Periodic Table of Elements PowerPoint Presentation, free Are Metalloids Dull And Brittle Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metalloids typically have several forms or. Silicon is a metalloid because it has luster, but is brittle. Metalloids have semiconductor properties and form amphoteric oxides. Properties of. Are Metalloids Dull And Brittle.
From www.slideshare.net
The Periodic Table Are Metalloids Dull And Brittle Silicon for example appears lustrous, but is. Silicon is a metalloid because it has luster, but is brittle. Metalloids typically have several forms or. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids.. Are Metalloids Dull And Brittle.
From slideplayer.com
Naming and Classifying the Elements ppt download Are Metalloids Dull And Brittle Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Properties of metalloids or semimetals. Silicon is a metalloid because it has luster, but is brittle. Silicon for example appears lustrous, but is. Metalloids have semiconductor properties and form amphoteric oxides. Metalloids or semimetals possess some. Are Metalloids Dull And Brittle.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Are Metalloids Dull And Brittle Silicon for example appears lustrous, but is. Metalloids typically have several forms or. Properties of metalloids or semimetals. Metalloids or semimetals possess some properties of metals and some of nonmetals. Location on the periodic table. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids. Are Metalloids Dull And Brittle.
From www.slideserve.com
PPT The Periodic Table of Elements PowerPoint Presentation, free Are Metalloids Dull And Brittle Metalloids typically have several forms or. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they. Are Metalloids Dull And Brittle.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Are Metalloids Dull And Brittle Location on the periodic table. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids typically have several forms or. Silicon is a metalloid because it has. Are Metalloids Dull And Brittle.
From slideplayer.com
Metals, NonMetals, Metalloids ppt download Are Metalloids Dull And Brittle Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids have semiconductor properties and form amphoteric oxides. Silicon is a metalloid because it has luster, but is brittle. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metalloids typically. Are Metalloids Dull And Brittle.
From homedeso.vercel.app
Metals And Metalloids On Periodic Table Are Metalloids Dull And Brittle Metalloids or semimetals possess some properties of metals and some of nonmetals. Silicon is a metalloid because it has luster, but is brittle. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metalloids have semiconductor properties and form amphoteric oxides. Most metalloids have a shiny, metallic appearance but. Are Metalloids Dull And Brittle.
From slideplayer.com
Topic 3 Elements & the Periodic Table ppt download Are Metalloids Dull And Brittle Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Location on the periodic table. Properties of metalloids or semimetals. Metalloids typically have several forms or. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Some metalloids,. Are Metalloids Dull And Brittle.
From slideplayer.com
The Periodic Table & Periodic Law. ppt download Are Metalloids Dull And Brittle Silicon is a metalloid because it has luster, but is brittle. Metalloids or semimetals possess some properties of metals and some of nonmetals. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids have semiconductor properties and form amphoteric oxides. Metals tend to be lustrous and shiny, but nonmetals tend to. Are Metalloids Dull And Brittle.
From slideplayer.com
Periodic Table Chapter ppt download Are Metalloids Dull And Brittle Location on the periodic table. Metalloids have semiconductor properties and form amphoteric oxides. Silicon for example appears lustrous, but is. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions,. Are Metalloids Dull And Brittle.
From www.slideserve.com
PPT Metal, Nonmetal or Metalloid? PowerPoint Presentation, free Are Metalloids Dull And Brittle Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Location on the periodic table. Metalloids have semiconductor properties and form amphoteric oxides. Silicon for example appears lustrous, but is. Metalloids or semimetals possess some properties of metals and some of nonmetals. Properties of metalloids or semimetals. Metalloids typically. Are Metalloids Dull And Brittle.
From slideplayer.com
Periodic Table History ppt download Are Metalloids Dull And Brittle Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Silicon for example appears lustrous, but is. Silicon is a metalloid because it has luster,. Are Metalloids Dull And Brittle.
From slideplayer.com
The Periodic Table. Types of Elements Metals Shiny, good conductors Are Metalloids Dull And Brittle Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids have semiconductor properties and form amphoteric oxides. Properties of metalloids or semimetals. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Location on the periodic table.. Are Metalloids Dull And Brittle.
From www.slideserve.com
PPT Metals, Nonmetals and Metalloids PowerPoint Presentation, free Are Metalloids Dull And Brittle Silicon is a metalloid because it has luster, but is brittle. Metalloids typically have several forms or. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form. Are Metalloids Dull And Brittle.
From slideplayer.com
Periodic Table History ppt download Are Metalloids Dull And Brittle Silicon is a metalloid because it has luster, but is brittle. Silicon for example appears lustrous, but is. Properties of metalloids or semimetals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Location on the periodic table. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional. Are Metalloids Dull And Brittle.
From slideplayer.com
Unit 5 The Periodic Table Section 1 Organizing the Elements ppt Are Metalloids Dull And Brittle Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metalloids or semimetals possess some properties of metals and some of nonmetals. Location on the periodic table. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form. Are Metalloids Dull And Brittle.
From www.slideserve.com
PPT The Periodic Table of Elements PowerPoint Presentation, free Are Metalloids Dull And Brittle Silicon is a metalloid because it has luster, but is brittle. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Properties of metalloids or semimetals. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called. Are Metalloids Dull And Brittle.
From www.slideserve.com
PPT 2.2 The Periodic table and Chemical Properties PowerPoint Are Metalloids Dull And Brittle Silicon for example appears lustrous, but is. Location on the periodic table. Metalloids have semiconductor properties and form amphoteric oxides. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids typically have several forms or. Metalloids or semimetals possess some properties of metals and some. Are Metalloids Dull And Brittle.
From slideplayer.com
Elements & the Periodic Table ppt download Are Metalloids Dull And Brittle Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids typically have several forms or. Silicon for example appears lustrous, but is. Location on the periodic table.. Are Metalloids Dull And Brittle.
From www.numerade.com
SOLVED Which properties do metalloids share with metals? PER Both are Are Metalloids Dull And Brittle Metalloids have semiconductor properties and form amphoteric oxides. Silicon is a metalloid because it has luster, but is brittle. Properties of metalloids or semimetals. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids or semimetals possess some properties of metals and some of nonmetals.. Are Metalloids Dull And Brittle.
From slideplayer.com
Elements Groups/Families ppt download Are Metalloids Dull And Brittle Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids typically have several forms or. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metalloids or semimetals possess some properties of metals and some of nonmetals. Silicon is a. Are Metalloids Dull And Brittle.
From slideplayer.com
Introduction to the Periodic Table ppt download Are Metalloids Dull And Brittle Metalloids have semiconductor properties and form amphoteric oxides. Metalloids or semimetals possess some properties of metals and some of nonmetals. Silicon for example appears lustrous, but is. Location on the periodic table. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids typically have several. Are Metalloids Dull And Brittle.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Are Metalloids Dull And Brittle Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Properties of metalloids or semimetals. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Some metalloids, such as silicon and germanium, can act as electrical conductors under. Are Metalloids Dull And Brittle.
From slideplayer.com
Lesson Objective You will be able to define “metalloids” and be able Are Metalloids Dull And Brittle Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metalloids have semiconductor properties and form amphoteric oxides. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic. Are Metalloids Dull And Brittle.
From slideplayer.com
Physical Science SPEEDY Midterm Review ppt download Are Metalloids Dull And Brittle Metalloids typically have several forms or. Location on the periodic table. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Silicon for example appears lustrous, but is. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metalloids have semiconductor properties and form amphoteric oxides. Silicon. Are Metalloids Dull And Brittle.
From www.youtube.com
Metals, Nonmetals and Metalloids. Ductile, Brittle. Shiny, Dull. Boron Are Metalloids Dull And Brittle Silicon for example appears lustrous, but is. Metalloids have semiconductor properties and form amphoteric oxides. Metalloids or semimetals possess some properties of metals and some of nonmetals. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Location on the periodic table. Silicon is a metalloid. Are Metalloids Dull And Brittle.
From www.difference101.com
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons Are Metalloids Dull And Brittle Location on the periodic table. Metalloids typically have several forms or. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Most metalloids have a. Are Metalloids Dull And Brittle.
From slideplayer.com
Ch 2 Matter and Change. ppt download Are Metalloids Dull And Brittle Silicon for example appears lustrous, but is. Location on the periodic table. Most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Silicon is a metalloid because it has luster, but is brittle. Metalloids or semimetals possess some properties of metals and some of nonmetals. Some metalloids, such as silicon and germanium,. Are Metalloids Dull And Brittle.
From slideplayer.com
Matter. ppt download Are Metalloids Dull And Brittle Silicon is a metalloid because it has luster, but is brittle. Location on the periodic table. Silicon for example appears lustrous, but is. Metalloids or semimetals possess some properties of metals and some of nonmetals. Properties of metalloids or semimetals. Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when. Are Metalloids Dull And Brittle.
From www.adda247.com
What are Metalloids? Definition, Properties and Example Are Metalloids Dull And Brittle Metals tend to be lustrous and shiny, but nonmetals tend to be transparent when they are gases and dull when they form brittle solids. Silicon for example appears lustrous, but is. Some metalloids, such as silicon and germanium, can act as electrical conductors under the right conditions, thus they are called semiconductors. Metalloids or semimetals possess some properties of metals. Are Metalloids Dull And Brittle.