What Is The Bromine Ions Net Charge After This Change . as you have learned, ions are atoms or molecules bearing an electrical charge. The charge that an atom acquires when it becomes an ion is related to the structure of the periodic. atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. atoms of group 17 gain one electron and form anions with a 1− charge; A cation (a positive ion) forms when a. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. The balanced equation will be calculated along. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). enter an equation of an ionic chemical equation and press the balance button.
from fyoiybdke.blob.core.windows.net
The balanced equation will be calculated along. atoms of group 17 gain one electron and form anions with a 1− charge; as you have learned, ions are atoms or molecules bearing an electrical charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The charge that an atom acquires when it becomes an ion is related to the structure of the periodic. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. A cation (a positive ion) forms when a. atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance.
Bromine Atomic Ion at Rebecca Clewis blog
What Is The Bromine Ions Net Charge After This Change atoms of group 17 gain one electron and form anions with a 1− charge; atoms of group 17 gain one electron and form anions with a 1− charge; The charge that an atom acquires when it becomes an ion is related to the structure of the periodic. The balanced equation will be calculated along. A cation (a positive ion) forms when a. atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. enter an equation of an ionic chemical equation and press the balance button. as you have learned, ions are atoms or molecules bearing an electrical charge.
From material-properties.org
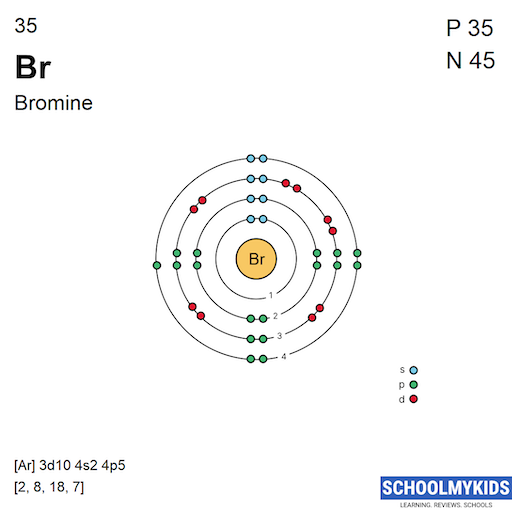
Bromine Periodic Table and Atomic Properties What Is The Bromine Ions Net Charge After This Change The charge that an atom acquires when it becomes an ion is related to the structure of the periodic. enter an equation of an ionic chemical equation and press the balance button. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). atoms of group 17 gain one. What Is The Bromine Ions Net Charge After This Change.
From exywfckgn.blob.core.windows.net
Bromine Ion Notation at Josephine Steward blog What Is The Bromine Ions Net Charge After This Change A cation (a positive ion) forms when a. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. The balanced equation will be calculated along. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). as you have learned, ions are atoms. What Is The Bromine Ions Net Charge After This Change.
From www.numerade.com
SOLVED 23. What happens when a bromine atom a bromide ion? (A What Is The Bromine Ions Net Charge After This Change atoms of group 17 gain one electron and form anions with a 1− charge; when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. as you have learned, ions are atoms or molecules bearing an electrical charge. When the atom loses or gains one or more electrons, the. What Is The Bromine Ions Net Charge After This Change.
From www.coursehero.com
[Solved] The formal charge on the bromine atom in BrO 3 drawn with What Is The Bromine Ions Net Charge After This Change atoms of group 17 gain one electron and form anions with a 1− charge; atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. The charge that an. What Is The Bromine Ions Net Charge After This Change.
From www.animalia-life.club
Electron Configuration For Bromine What Is The Bromine Ions Net Charge After This Change The balanced equation will be calculated along. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. . What Is The Bromine Ions Net Charge After This Change.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry What Is The Bromine Ions Net Charge After This Change A cation (a positive ion) forms when a. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. when forming ions, elements typically gain or lose the minimum number of electrons necessary. What Is The Bromine Ions Net Charge After This Change.
From slideplayer.com
Ionic charge and electrical conduction ppt download What Is The Bromine Ions Net Charge After This Change atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. enter an equation of an ionic chemical equation and press the balance button. A cation (a positive ion) forms when a. Atoms of group 16 gain two electrons and form ions with a 2−. What Is The Bromine Ions Net Charge After This Change.
From giovnhbbj.blob.core.windows.net
Chlorine And Bromine Ionic Or Covalent at Marjorie Dalton blog What Is The Bromine Ions Net Charge After This Change as you have learned, ions are atoms or molecules bearing an electrical charge. atoms of group 17 gain one electron and form anions with a 1− charge; atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. When the atom loses or gains. What Is The Bromine Ions Net Charge After This Change.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) What Is The Bromine Ions Net Charge After This Change The balanced equation will be calculated along. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. atoms that lose electrons acquire a positive charge because they are left with fewer negatively. What Is The Bromine Ions Net Charge After This Change.
From fyoiybdke.blob.core.windows.net
Bromine Atomic Ion at Rebecca Clewis blog What Is The Bromine Ions Net Charge After This Change Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The balanced equation will be calculated along. atoms that lose electrons acquire a positive charge because they are left with fewer negatively. What Is The Bromine Ions Net Charge After This Change.
From exyuexcnt.blob.core.windows.net
Bromine Atom Change at Ella Demars blog What Is The Bromine Ions Net Charge After This Change Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. as you have learned, ions are atoms or molecules bearing an electrical charge. enter an equation of an ionic chemical equation and press the balance button. A cation (a positive ion) forms when a. atoms that lose electrons acquire a. What Is The Bromine Ions Net Charge After This Change.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation What Is The Bromine Ions Net Charge After This Change enter an equation of an ionic chemical equation and press the balance button. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. The charge that an atom acquires when it becomes an ion is related to the structure of the periodic. A cation. What Is The Bromine Ions Net Charge After This Change.
From giomseqga.blob.core.windows.net
Bromine Charge And Symbol at Derek Windsor blog What Is The Bromine Ions Net Charge After This Change atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). A cation (a positive ion) forms when a. when forming ions, elements typically gain or lose the minimum number. What Is The Bromine Ions Net Charge After This Change.
From exyuexcnt.blob.core.windows.net
Bromine Atom Change at Ella Demars blog What Is The Bromine Ions Net Charge After This Change atoms of group 17 gain one electron and form anions with a 1− charge; enter an equation of an ionic chemical equation and press the balance button. atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. Atoms of group 16 gain two electrons and form ions with. What Is The Bromine Ions Net Charge After This Change.
From giohkxlsl.blob.core.windows.net
Bromine Common Ion Formed at Deborah McAndrews blog What Is The Bromine Ions Net Charge After This Change enter an equation of an ionic chemical equation and press the balance button. atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. The charge that an atom acquires when it becomes. What Is The Bromine Ions Net Charge After This Change.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram What Is The Bromine Ions Net Charge After This Change when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. The charge that an atom acquires when it becomes an ion is related to the structure of the periodic. . What Is The Bromine Ions Net Charge After This Change.
From www.animalia-life.club
Electron Configuration For Bromine What Is The Bromine Ions Net Charge After This Change atoms of group 17 gain one electron and form anions with a 1− charge; atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The balanced equation will be. What Is The Bromine Ions Net Charge After This Change.
From fyopvzszf.blob.core.windows.net
Bromine Chloride Electronic Structure at Thomas Steed blog What Is The Bromine Ions Net Charge After This Change atoms of group 17 gain one electron and form anions with a 1− charge; The balanced equation will be calculated along. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. atoms that lose electrons acquire a positive charge because they are left. What Is The Bromine Ions Net Charge After This Change.
From giomwhfig.blob.core.windows.net
Bromine Equation Electron at Alexis Barnhart blog What Is The Bromine Ions Net Charge After This Change Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. as you have learned, ions are atoms or molecules bearing an electrical charge. The charge that an atom acquires when it becomes an ion is related to the structure of the periodic. enter an equation of an ionic chemical equation and. What Is The Bromine Ions Net Charge After This Change.
From exywfckgn.blob.core.windows.net
Bromine Ion Notation at Josephine Steward blog What Is The Bromine Ions Net Charge After This Change atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. enter an equation of an ionic chemical equation and press the balance button. The. What Is The Bromine Ions Net Charge After This Change.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube What Is The Bromine Ions Net Charge After This Change Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). as you have learned, ions are atoms or molecules bearing an electrical charge. The balanced equation will be calculated along. A cation. What Is The Bromine Ions Net Charge After This Change.
From www.numerade.com
SOLVED ion 7 What is the formal charge on nitrogen in NOz" ? (hint What Is The Bromine Ions Net Charge After This Change The charge that an atom acquires when it becomes an ion is related to the structure of the periodic. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). atoms of group 17 gain one electron and form anions with a 1− charge; atoms of the elements display. What Is The Bromine Ions Net Charge After This Change.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube What Is The Bromine Ions Net Charge After This Change atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. enter an equation of an ionic chemical equation and press the balance button. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Atoms. What Is The Bromine Ions Net Charge After This Change.
From h-o-m-e.org
Bromide (Br) Ion Charges Explained What Is The Bromine Ions Net Charge After This Change atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. When the atom loses or gains one or more electrons, the electric charge is generated. What Is The Bromine Ions Net Charge After This Change.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) What Is The Bromine Ions Net Charge After This Change enter an equation of an ionic chemical equation and press the balance button. A cation (a positive ion) forms when a. The balanced equation will be calculated along. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. atoms of the elements display a range of charges, but you can predict. What Is The Bromine Ions Net Charge After This Change.
From exyvvdwam.blob.core.windows.net
Bromine Ion Bohr Diagram at Craig Carvajal blog What Is The Bromine Ions Net Charge After This Change atoms of group 17 gain one electron and form anions with a 1− charge; when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group.. What Is The Bromine Ions Net Charge After This Change.
From quizdbcornwallis.z21.web.core.windows.net
What Is Bromine Charge What Is The Bromine Ions Net Charge After This Change atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. as you have learned, ions are atoms or molecules bearing an electrical charge. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. . What Is The Bromine Ions Net Charge After This Change.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine What Is The Bromine Ions Net Charge After This Change The balanced equation will be calculated along. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The charge that an atom acquires when it becomes an ion is related to the structure of the periodic. When the atom loses or gains one or more electrons, the electric charge is. What Is The Bromine Ions Net Charge After This Change.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements What Is The Bromine Ions Net Charge After This Change The balanced equation will be calculated along. as you have learned, ions are atoms or molecules bearing an electrical charge. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. A cation (a positive ion) forms when a. atoms of the elements display a range of charges, but you can predict. What Is The Bromine Ions Net Charge After This Change.
From exywfckgn.blob.core.windows.net
Bromine Ion Notation at Josephine Steward blog What Is The Bromine Ions Net Charge After This Change enter an equation of an ionic chemical equation and press the balance button. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. When the atom loses or gains one or more. What Is The Bromine Ions Net Charge After This Change.
From enginelistjuprefecture.z21.web.core.windows.net
Lewis Diagram For Bromine What Is The Bromine Ions Net Charge After This Change atoms of group 17 gain one electron and form anions with a 1− charge; when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. as you have learned, ions are atoms or molecules bearing an electrical charge. Atoms of group 16 gain two electrons and form ions with. What Is The Bromine Ions Net Charge After This Change.
From ar.inspiredpencil.com
Electron Configuration For Bromine What Is The Bromine Ions Net Charge After This Change atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. A cation (a positive ion) forms when a. When the atom loses or gains one or more electrons, the electric. What Is The Bromine Ions Net Charge After This Change.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of What Is The Bromine Ions Net Charge After This Change atoms of group 17 gain one electron and form anions with a 1− charge; when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group.. What Is The Bromine Ions Net Charge After This Change.
From www.numerade.com
SOLVED Predict the common charge of the bromine ion A 2+ B. I+ C. 3+ D What Is The Bromine Ions Net Charge After This Change A cation (a positive ion) forms when a. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The balanced equation will be calculated along. atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group.. What Is The Bromine Ions Net Charge After This Change.
From giohkxlsl.blob.core.windows.net
Bromine Common Ion Formed at Deborah McAndrews blog What Is The Bromine Ions Net Charge After This Change atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. A cation (a positive ion) forms when a. The charge that an atom acquires when it becomes an. What Is The Bromine Ions Net Charge After This Change.