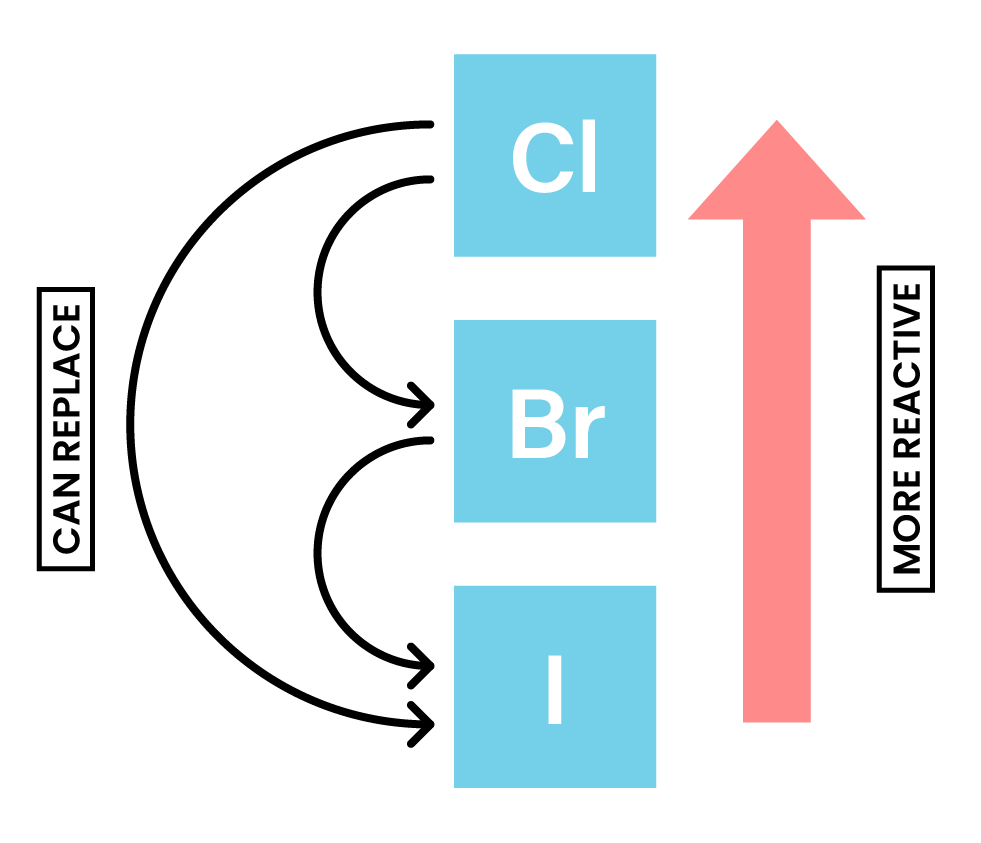
Bromine Iodide Colour . since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. However, bromides and iodides develop colour when exposed to light. The order of reactivity is. alkyl halides are colourless when pure. You might need a white background to see the colour of the chlorine solution. bromine is a chemical element; It has symbol br and atomic number 35. halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. for chlorine and bromine the colour does not change.
from resource.studiaacademy.com
use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. bromine is a chemical element; alkyl halides are colourless when pure. You might need a white background to see the colour of the chlorine solution. The order of reactivity is. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. It has symbol br and atomic number 35. However, bromides and iodides develop colour when exposed to light.
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy
Bromine Iodide Colour for chlorine and bromine the colour does not change. You might need a white background to see the colour of the chlorine solution. It has symbol br and atomic number 35. alkyl halides are colourless when pure. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. The order of reactivity is. for chlorine and bromine the colour does not change. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. However, bromides and iodides develop colour when exposed to light. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. bromine is a chemical element; halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement.
From www.youtube.com
Bromine Water + Sodium Iodide YouTube Bromine Iodide Colour use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. for chlorine and bromine the colour does not change. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. However, for iodine there is a colour change, from brown. Bromine Iodide Colour.
From slideplayer.com
Chemsheets AS006 (Electron arrangement) ppt download Bromine Iodide Colour use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. However, bromides and iodides develop colour when exposed to light. alkyl halides are colourless when pure. You might need a white background to see the colour of the chlorine solution. when bromine is added to the sodium iodide solution,. Bromine Iodide Colour.
From www.bigstockphoto.com
Test Bromides Iodides Image & Photo (Free Trial) Bigstock Bromine Iodide Colour since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. You might need a white background to see the colour of the chlorine solution. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2. Bromine Iodide Colour.
From www.slideshare.net
Oxidation & reduction Bromine Iodide Colour You might need a white background to see the colour of the chlorine solution. It has symbol br and atomic number 35. for chlorine and bromine the colour does not change. The order of reactivity is. alkyl halides are colourless when pure. halogen solutions are coloured and sometimes it can be difficult to determine colour changes during. Bromine Iodide Colour.
From quizlet.com
OxidationReduction Experiments Addition of Bromine Water to a Bromine Iodide Colour bromine is a chemical element; for chlorine and bromine the colour does not change. You might need a white background to see the colour of the chlorine solution. The order of reactivity is. halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. use the results in the table to. Bromine Iodide Colour.
From alannameowarias.blogspot.com
Colour of Bromine Water Bromine Iodide Colour use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)). Bromine Iodide Colour.
From www.slideshare.net
Reactions Of The Halogens (2) Bromine Iodide Colour It has symbol br and atomic number 35. The order of reactivity is. halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. bromine is a chemical element; when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i. Bromine Iodide Colour.
From brainly.in
Reaction between sodium iodide and bromine water Brainly.in Bromine Iodide Colour halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. The order of reactivity is. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. bromine is a chemical element; You might need a white background to see the colour of. Bromine Iodide Colour.
From www.youtube.com
Bromine and potassium bromide YouTube Bromine Iodide Colour halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. However, bromides and iodides develop colour when exposed to light. since the partition coefficient of iodine or bromine is much higher for the organic. Bromine Iodide Colour.
From www.youtube.com
Iodine + Sodium Bromide YouTube Bromine Iodide Colour It has symbol br and atomic number 35. for chlorine and bromine the colour does not change. The order of reactivity is. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr. Bromine Iodide Colour.
From www.compoundchem.com
Compound Interest Chemical Reactions Lead Iodide & ‘Golden Rain’ Bromine Iodide Colour since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. You might need a white background to see the colour of the chlorine solution. for chlorine and bromine the colour does not change. halogen solutions are coloured and sometimes it can be difficult to determine colour changes. Bromine Iodide Colour.
From joelgordon.photoshelter.com
Chlorine Bromine Iodine Joel Gordon Photography Bromine Iodide Colour when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. halogen solutions are coloured and sometimes it can be difficult to. Bromine Iodide Colour.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Bromine Iodide Colour bromine is a chemical element; since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. for chlorine and bromine the colour does not change. You might need a white background to see the colour of the chlorine solution. halogen solutions are coloured and sometimes it can. Bromine Iodide Colour.
From www.differencebetween.com
Difference Between Bromine and Iodine Compare the Difference Between Bromine Iodide Colour bromine is a chemical element; halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide. Bromine Iodide Colour.
From dxoswjfuw.blob.core.windows.net
Bromine Colour In Solution at Doris Wash blog Bromine Iodide Colour use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. alkyl halides are colourless when pure. It has symbol br and atomic number. Bromine Iodide Colour.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Bromine Iodide Colour use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The order of reactivity is. alkyl halides are colourless when pure. However, bromides and iodides develop colour when exposed to light. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. . Bromine Iodide Colour.
From ar.inspiredpencil.com
Bromine Water Colour Bromine Iodide Colour It has symbol br and atomic number 35. However, bromides and iodides develop colour when exposed to light. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. bromine is a chemical element; since the partition coefficient of iodine or bromine. Bromine Iodide Colour.
From www.youtube.com
Bromine Water + Sodium Chloride YouTube Bromine Iodide Colour However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. alkyl halides are colourless when pure. bromine is a chemical element; since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. use the results in the table to. Bromine Iodide Colour.
From exowuxaix.blob.core.windows.net
Bromine Colour Gas at Jennie Marko blog Bromine Iodide Colour halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. It has symbol br and atomic number 35. However, bromides and iodides develop colour when exposed to light. However, for iodine there. Bromine Iodide Colour.
From exoicnyjk.blob.core.windows.net
Properties Of Halogens Gas at Lora Maynes blog Bromine Iodide Colour bromine is a chemical element; It has symbol br and atomic number 35. halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. since the partition coefficient of iodine or bromine is much. Bromine Iodide Colour.
From periodictable.com
Cobalt bromide. , a sample of the element Bromine in the Periodic Table Bromine Iodide Colour alkyl halides are colourless when pure. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. You might need a white background to see the colour of the chlorine solution. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer.. Bromine Iodide Colour.
From www.shalom-education.com
Testing for Halide ions GCSE Chemistry Revision Bromine Iodide Colour when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. The order of reactivity is. bromine is a chemical element; You might need a white background to see the colour of the chlorine solution. since the partition coefficient of iodine or. Bromine Iodide Colour.
From fphoto.photoshelter.com
science chemistry iodine solution Fundamental Photographs The Art Bromine Iodide Colour since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. alkyl halides are colourless when pure. The order of reactivity is. for chlorine and bromine the colour does not change. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon. Bromine Iodide Colour.
From www.coursehero.com
[Solved] Bromine water (golden yellow solution) and the purple Bromine Iodide Colour when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. You might need a white background to see the colour of the chlorine solution. However, bromides. Bromine Iodide Colour.
From www.slideshare.net
Redox Bromine Iodide Colour bromine is a chemical element; However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. However, bromides and iodides develop colour when exposed to light. You might need a white background to see the colour of the chlorine solution. alkyl halides are colourless when pure. It has symbol br and. Bromine Iodide Colour.
From www.coursehero.com
Bromine water (golden yellow solution) and the purple potassium Bromine Iodide Colour when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. You might need a white background to see the colour of the chlorine solution.. Bromine Iodide Colour.
From www.youtube.com
Bromine and potassium iodide YouTube Bromine Iodide Colour when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. alkyl halides are colourless when pure. for chlorine and bromine. Bromine Iodide Colour.
From www.youtube.com
Bromine and potassium iodide YouTube Bromine Iodide Colour use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. bromine is a chemical element; since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. However, bromides and iodides develop colour when exposed to light. It has symbol br. Bromine Iodide Colour.
From fineartamerica.com
Bromine Test For Alkene Photograph by Andrew Lambert Photography Bromine Iodide Colour You might need a white background to see the colour of the chlorine solution. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. bromine is a chemical element; for chlorine and bromine the colour does not change. halogen solutions are coloured and sometimes it can. Bromine Iodide Colour.
From www.bbc.co.uk
What is a displacement reaction? BBC Bitesize Bromine Iodide Colour However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. for chlorine and bromine the colour does not change. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The order of reactivity is. You might need a white background to see. Bromine Iodide Colour.
From quintensrtate.blogspot.com
Colour of Bromine Water QuintensrTate Bromine Iodide Colour However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. alkyl halides are colourless when pure. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. The order of reactivity is. bromine is. Bromine Iodide Colour.
From fphoto.photoshelter.com
halogens chemistry bromine chlorine iodine Fundamental Photographs Bromine Iodide Colour since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. However, for iodine there is a colour change, from brown in water to purple in the hydrocarbon layer. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and. Bromine Iodide Colour.
From www.livescience.com
Facts About Bromine Live Science Bromine Iodide Colour It has symbol br and atomic number 35. You might need a white background to see the colour of the chlorine solution. bromine is a chemical element; The order of reactivity is. alkyl halides are colourless when pure. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is. Bromine Iodide Colour.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Bromine Iodide Colour alkyl halides are colourless when pure. It has symbol br and atomic number 35. The order of reactivity is. halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. since the partition coefficient of iodine or bromine is much higher for the organic solvent, a colored layer is formed. However, bromides. Bromine Iodide Colour.
From www.numerade.com
SOLVED Bromine displaces iodine from sodium iodide, but there is no Bromine Iodide Colour halogen solutions are coloured and sometimes it can be difficult to determine colour changes during displacement. for chlorine and bromine the colour does not change. when bromine is added to the sodium iodide solution, a brown colour is seen, as sodium bromide (nabr (aq)) and brown iodine (i 2 (aq)) are. However, bromides and iodides develop colour. Bromine Iodide Colour.