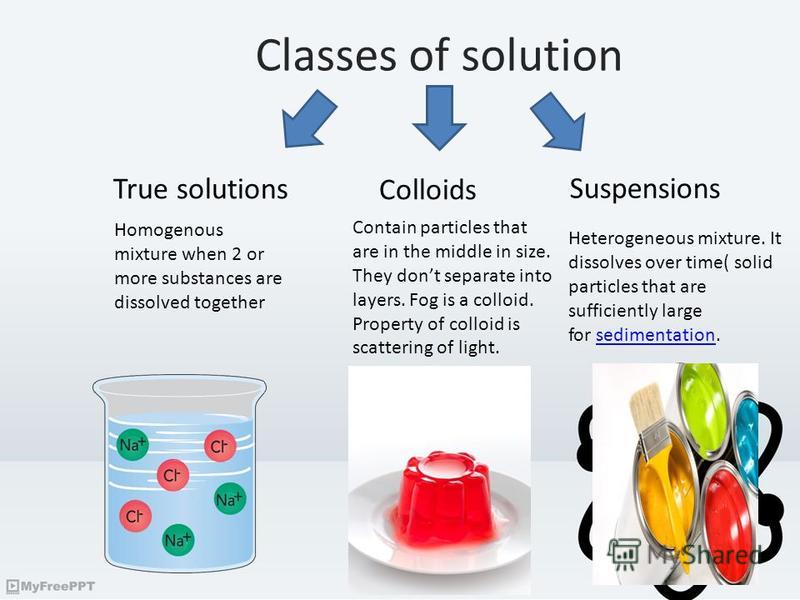
Suspension Colloid . Colloids have particles that are. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The main difference between colloid and suspension lies in the size of particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Colloid particles are much smaller than suspension particles. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A solution is a homogeneous mixture of.
from www.myshared.ru
Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Colloids have particles that are. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. The main difference between colloid and suspension lies in the size of particles. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A solution is a homogeneous mixture of.
Презентация на тему "Colloidal systems. Classes of solution True
Suspension Colloid Colloids have particles that are. A solution is a homogeneous mixture of. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. The main difference between colloid and suspension lies in the size of particles. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Colloid particles are much smaller than suspension particles. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Colloids have particles that are. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1.
From studylib.net
Solutions, Suspensions & Colloids Suspension Colloid Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Colloid particles are much smaller than suspension particles. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. The. Suspension Colloid.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Suspension Colloid Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics. Suspension Colloid.
From www.researchgate.net
Schematic illustration of a colloidal suspension of silica spheres in Suspension Colloid The main difference between colloid and suspension lies in the size of particles. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Suspensions are considered heterogeneous because the different substances in the mixture will not. Suspension Colloid.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Suspension Colloid Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A solution is a homogeneous mixture of. The main difference between colloid and suspension lies in the size of particles. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. The particles are. Suspension Colloid.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension Colloid The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. The main difference between colloid and suspension lies in the size of particles. A solution is a homogeneous mixture of. Colloid particles are. Suspension Colloid.
From askanydifference.com
Colloid vs Suspension Difference and Comparison Suspension Colloid In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. A solution is a homogeneous mixture of. The main difference between colloid and suspension lies in the size of particles. Solutions, suspensions, colloids, and other. Suspension Colloid.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Suspension Colloid In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. Colloid particles are much smaller than suspension. Suspension Colloid.
From www.sliderbase.com
Suspensions and Colloids Presentation Chemistry Suspension Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others.. Suspension Colloid.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension Colloid The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A solution is a homogeneous mixture of. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium.. Suspension Colloid.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension Colloid A solution is a homogeneous mixture of. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. In chemistry, a colloid is a mixture of tiny particles. Suspension Colloid.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Suspension Colloid Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics. Suspension Colloid.
From www.youtube.com
Science 6 Q1 Week 2 Solution, Suspension, Colloid YouTube Suspension Colloid In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids have particles that are. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. Suspensions and. Suspension Colloid.
From www.nagwa.com
Vidéo de la leçon Colloïdes et les suspensions Nagwa Suspension Colloid A solution is a homogeneous mixture of. Colloid particles are much smaller than suspension particles. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Colloids have particles that are. The main difference between colloid and suspension lies. Suspension Colloid.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Suspension Colloid The main difference between colloid and suspension lies in the size of particles. Colloids have particles that are. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Suspensions and colloids are two common. Suspension Colloid.
From www.thoughtco.com
Solutions, Suspensions, Colloids, and Dispersions Suspension Colloid Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Colloid particles are much smaller than suspension particles. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Colloids and suspensions. Suspension Colloid.
From www.youtube.com
Suspension Colloidal solution Science Class 9 Chapter 2 (Part 4 Suspension Colloid Colloid particles are much smaller than suspension particles. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Colloids have particles that are. Colloids and suspensions are both types of mixtures, but they differ in terms of. Suspension Colloid.
From chemistry-examples-00.blogspot.com
8 EXAMPLES OF SUSPENSION COLLOID AND SOLUTION, SUSPENSION OF SOLUTION Suspension Colloid A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. A solution is a homogeneous mixture of. Colloids have particles that are. Colloid particles are much smaller than suspension particles. The main difference between colloid. Suspension Colloid.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Colloid Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids have particles that are. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. A solution is. Suspension Colloid.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Suspension Colloid Colloids have particles that are. Colloid particles are much smaller than suspension particles. The main difference between colloid and suspension lies in the size of particles. A solution is a homogeneous mixture of. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. In chemistry, a. Suspension Colloid.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. The main difference between colloid and suspension lies in the size of particles. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. In chemistry, a colloid. Suspension Colloid.
From www.pinterest.com
solutionssuspensionsandcolloids9728.jpg (728×546) Science Suspension Colloid The main difference between colloid and suspension lies in the size of particles. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly. Suspension Colloid.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Suspension Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. Colloid particles are much smaller than suspension particles. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids have particles that are. A solution is a homogeneous mixture of.. Suspension Colloid.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Suspension Colloid The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Colloid particles are much smaller than suspension particles. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Suspensions are. Suspension Colloid.
From stock.adobe.com
True Solution, Colloid solution and Suspension three different types of Suspension Colloid A solution is a homogeneous mixture of. Colloids have particles that are. Colloid particles are much smaller than suspension particles. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Solutions, suspensions, colloids, and. Suspension Colloid.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspension Colloid Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. Colloids have particles that are. The main difference between colloid and suspension lies in the size of particles. Suspensions and colloids are. Suspension Colloid.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Suspension Colloid A solution is a homogeneous mixture of. Colloids have particles that are. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Colloid particles are much smaller than suspension particles. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and. Suspension Colloid.
From www.aquaportail.com
Solution colloïdale définition et explications Suspension Colloid Colloid particles are much smaller than suspension particles. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. In chemistry,. Suspension Colloid.
From www.youtube.com
23 Suspensions and colloids (1st year secondary first term) YouTube Suspension Colloid The main difference between colloid and suspension lies in the size of particles. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloid particles are much smaller than suspension particles. Suspensions are considered heterogeneous because the different substances in the mixture will not remain uniformly distributed if they are. Colloids have particles that. Suspension Colloid.
From www.slideserve.com
PPT Colloids, Solutions, Suspensions PowerPoint Presentation, free Suspension Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. The main difference between colloid and suspension lies in the size of particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Colloid particles are much smaller than. Suspension Colloid.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Suspension Colloid In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The main difference between colloid and suspension lies in the size of particles. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set. Suspension Colloid.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspension Colloid A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. A solution is a homogeneous mixture of. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Solutions, suspensions,. Suspension Colloid.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. Colloids and suspensions are both types of mixtures, but they differ in terms of particle size and stability. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. Suspensions. Suspension Colloid.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Suspension Colloid A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed. A solution is a homogeneous mixture of. Colloids have particles that are. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloid particles are much smaller than suspension particles. Colloids and suspensions are both types of. Suspension Colloid.
From www.youtube.com
Solution colloids n suspension class 9 by neelam dwivedi YouTube Suspension Colloid Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Suspensions are considered heterogeneous because the different. Suspension Colloid.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Suspension Colloid Colloid particles are much smaller than suspension particles. A solution is a homogeneous mixture of. Solutions, suspensions, colloids, and other dispersions are similar but have characteristics that set each one apart from the others. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of true solutions and heterogeneous mixtures. The particles. Suspension Colloid.