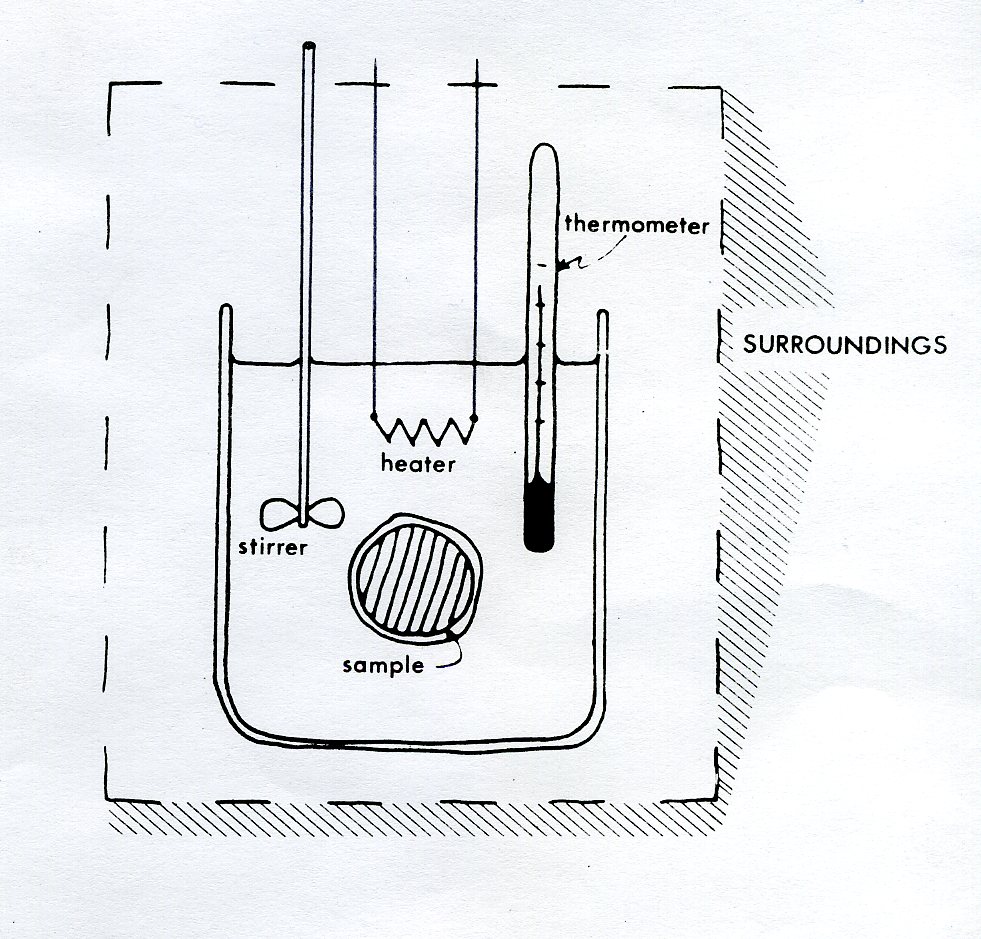
Calorimeter Formula Explanation . In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Solved examples of calorimetry formula. Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during. Calculate the heat transfer when 200. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) Calculate and interpret heat and related properties using typical calorimetry data.
from serc.carleton.edu
Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the heat transfer when 200. In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. Calculate and interpret heat and related properties using typical calorimetry data. 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during.
Calorimetry
Calorimeter Formula Explanation Calorimetry is used to measure amounts of heat transferred to or from a substance. In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during. The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is used to measure amounts of heat transferred to or from a substance. 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. Calculate the heat transfer when 200. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. Solved examples of calorimetry formula. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Formula Explanation 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimeter Formula Explanation.
From printablecampusreises.z21.web.core.windows.net
How To Calculate Calorimetry Problems Calorimeter Formula Explanation Calculate the heat transfer when 200. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calculate and interpret heat and related properties using typical calorimetry data. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05. Calorimeter Formula Explanation.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Formula Explanation Calculate and interpret heat and related properties using typical calorimetry data. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.. Calorimeter Formula Explanation.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Formula Explanation The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calculate and interpret heat and related properties. Calorimeter Formula Explanation.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Formula Explanation The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) Calculate the heat transfer when 200. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical calorimetry data.. Calorimeter Formula Explanation.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimeter Formula Explanation Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Solved examples of calorimetry formula. The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) Using the. Calorimeter Formula Explanation.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Formula Explanation 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. Calculate and interpret heat and related properties using typical. Calorimeter Formula Explanation.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Calorimeter Formula Explanation In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. Calculate and interpret heat and related properties using typical calorimetry data. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ). Calorimeter Formula Explanation.
From klaxzllai.blob.core.windows.net
Calorimetry Equation Examples at Grace Smallwood blog Calorimeter Formula Explanation Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during. 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. Using the calorimetry formula, you can calculate the heat gained by. Calorimeter Formula Explanation.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Formula Explanation Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during. Calculate the heat transfer when 200. Solved examples of calorimetry formula. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.. Calorimeter Formula Explanation.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Formula Explanation One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. Calorimetry is a branch of science concerned with measuring a body’s. Calorimeter Formula Explanation.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimeter Formula Explanation Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. One technique we can use to measure the amount. Calorimeter Formula Explanation.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Formula Explanation In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with. Calorimeter Formula Explanation.
From klaxzllai.blob.core.windows.net
Calorimetry Equation Examples at Grace Smallwood blog Calorimeter Formula Explanation The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In a simple calorimetry process, (a) heat, q, is. Calorimeter Formula Explanation.
From serc.carleton.edu
Calorimetry Calorimeter Formula Explanation The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) Calculate and interpret heat and related properties using typical calorimetry data. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. Calorimeter Formula Explanation.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimeter Formula Explanation 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. Calorimetry is used to measure amounts of heat transferred. Calorimeter Formula Explanation.
From dxoazusse.blob.core.windows.net
Food Calorimetry Equation at Jesse Freeman blog Calorimeter Formula Explanation Calculate the heat transfer when 200. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during. In a. Calorimeter Formula Explanation.
From fieghel9udworkshopfix.z13.web.core.windows.net
Symbol For Heat In Chemistry Calorimeter Formula Explanation One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) Using the calorimetry formula, you can calculate the heat. Calorimeter Formula Explanation.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Calorimeter Formula Explanation Calorimetry is used to measure amounts of heat transferred to or from a substance. In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05. Calorimeter Formula Explanation.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Formula Explanation The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. Using the calorimetry formula,. Calorimeter Formula Explanation.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Calorimeter Formula Explanation Calorimetry is used to measure amounts of heat transferred to or from a substance. 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. Calculate the heat transfer when 200. Calculate and interpret heat and related properties using typical calorimetry data. In a simple calorimetry process,. Calorimeter Formula Explanation.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimeter Formula Explanation Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during. Solved examples of calorimetry formula. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. Calculate and interpret. Calorimeter Formula Explanation.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Formula Explanation Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Calorimeter Formula Explanation.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter Formula Explanation The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when calculating the final answer, as is shown in eq 5.6.12) Calorimetry is used to measure amounts of heat transferred to or from a substance. In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the. Calorimeter Formula Explanation.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Calorimeter Formula Explanation 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during. In a simple calorimetry process, (a) heat, q, is transferred from. Calorimeter Formula Explanation.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Formula Explanation 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. Calculate and interpret heat and related properties using typical. Calorimeter Formula Explanation.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Formula Explanation One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is used to measure amounts of heat transferred to or from a substance.. Calorimeter Formula Explanation.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimeter Formula Explanation Calorimetry is used to measure amounts of heat transferred to or from a substance. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. The calorimeter constant term was not shown in the numerator of the video but was taken into consideration when. Calorimeter Formula Explanation.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Formula Explanation Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is the part of chemistry which is about the study of the quantity of heat which is absorbed or released with the surrounding during.. Calorimeter Formula Explanation.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Calorimeter Formula Explanation Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the heat transfer when 200. 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. The calorimeter constant term was not shown in the numerator of the video but was taken. Calorimeter Formula Explanation.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Formula Explanation 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Using the calorimetry formula, you can calculate the heat gained by the. Calorimeter Formula Explanation.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimeter Formula Explanation One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calculate the heat. Calorimeter Formula Explanation.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimeter Formula Explanation One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Using the calorimetry formula, you can calculate the heat gained by the calorimeter as q = mc∆t = (0.05 ) (4.184 ) (30.25 − 25 °c) =. The calorimeter constant term was not shown in the numerator of. Calorimeter Formula Explanation.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimeter Formula Explanation 7'16 youtube calculating the final temperature for the problem in example \(\pageindex{1}\) in a real calorimeter with a calorimeter constant of 36.0 o c. In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are at the same. Calculate the heat transfer when 200. Using the calorimetry. Calorimeter Formula Explanation.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Formula Explanation One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. In a simple calorimetry process, (a) heat, q, is transferred from the hot metal, m, to the cool water, w, until (b) both are. Calorimeter Formula Explanation.