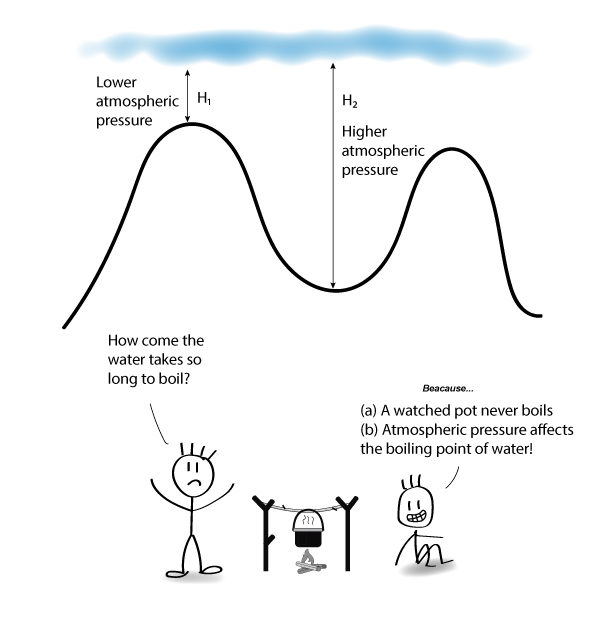
What Is Responsible For The Relatively High Boiling Point Of Pure Water . The most apparent peculiarity of water is its very high boiling point for such a light molecule. The boiling temperature of water decreases at higher elevations (lower. Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The “normal” refers to sea level or an elevation of 0 meters or feet. At sea level, pure water boils at 100 o c and freezes at o c. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid;
from surfguppy.com
Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The boiling temperature of water decreases at higher elevations (lower. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; The “normal” refers to sea level or an elevation of 0 meters or feet. The most apparent peculiarity of water is its very high boiling point for such a light molecule. At sea level, pure water boils at 100 o c and freezes at o c. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:
How does Atmospheric Pressure Affect Boiling Point
What Is Responsible For The Relatively High Boiling Point Of Pure Water The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The “normal” refers to sea level or an elevation of 0 meters or feet. The most apparent peculiarity of water is its very high boiling point for such a light molecule. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The boiling temperature of water decreases at higher elevations (lower. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; At sea level, pure water boils at 100 o c and freezes at o c.
From exobnyect.blob.core.windows.net
Why Does Steam Distillation Lower Boiling Point at Harold Sheehan blog What Is Responsible For The Relatively High Boiling Point Of Pure Water The boiling temperature of water decreases at higher elevations (lower. At sea level, pure water boils at 100 o c and freezes at o c. The most apparent peculiarity of water is its very high boiling point for such a light molecule. Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is Responsible For The Relatively High Boiling Point Of Pure Water Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The “normal” refers to sea level or an elevation of 0 meters or feet. At sea level, pure water boils at 100 o c and freezes at o c. Boiling point, temperature at which the pressure exerted by the surroundings upon a. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From pubchem.ncbi.nlm.nih.gov
Boiling Point Periodic Table of Elements PubChem What Is Responsible For The Relatively High Boiling Point Of Pure Water The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. At sea level, pure water boils at 100 o c and freezes at o c. The most apparent peculiarity of water is its very high boiling point for such a light molecule. The “normal” refers to sea level or an elevation of 0 meters or feet.. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is Responsible For The Relatively High Boiling Point Of Pure Water At sea level, pure water boils at 100 o c and freezes at o c. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The most apparent. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is Responsible For The Relatively High Boiling Point Of Pure Water The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The boiling temperature of water decreases at higher elevations (lower. Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The “normal” refers to sea level or an elevation of 0 meters. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.chegg.com
Solved suppose the boiling point of pure water at h h What Is Responsible For The Relatively High Boiling Point Of Pure Water The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The boiling temperature of water decreases at higher elevations (lower. The most apparent peculiarity of water is its very high boiling point for such a light molecule. At sea level, pure water boils at 100 o c and freezes at o c. Boiling point, temperature at. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From water-purifiers.com
How Pure Is Your Boiling Water What Is Responsible For The Relatively High Boiling Point Of Pure Water The “normal” refers to sea level or an elevation of 0 meters or feet. Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The most apparent peculiarity of water is its very high boiling point for such a light molecule. The boiling temperature of water. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From blog.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks What Is Responsible For The Relatively High Boiling Point Of Pure Water The most apparent peculiarity of water is its very high boiling point for such a light molecule. The boiling temperature of water decreases at higher elevations (lower. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is Responsible For The Relatively High Boiling Point Of Pure Water Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; The “normal” refers to sea level or an elevation of 0 meters or feet. Hydrogen bonds. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From fyocifxfr.blob.core.windows.net
Why Do You Put Salt In Water When Boiling at James Porter blog What Is Responsible For The Relatively High Boiling Point Of Pure Water The boiling temperature of water decreases at higher elevations (lower. Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The most apparent peculiarity of water is its very high boiling point for such a light molecule. Because water seems so ubiquitous, many people are unaware. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.alamy.com
Atmospheric pressure alters the boiling point of water Stock Photo Alamy What Is Responsible For The Relatively High Boiling Point Of Pure Water At sea level, pure water boils at 100 o c and freezes at o c. The most apparent peculiarity of water is its very high boiling point for such a light molecule. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The “normal” refers to sea level or an elevation of. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From exyigmeig.blob.core.windows.net
What Is Water's Normal Boiling Point at Lola Drummer blog What Is Responsible For The Relatively High Boiling Point Of Pure Water Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The boiling temperature of water decreases at higher elevations (lower. Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. Boiling point, temperature at which the pressure exerted. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country What Is Responsible For The Relatively High Boiling Point Of Pure Water The “normal” refers to sea level or an elevation of 0 meters or feet. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; The most apparent peculiarity of water is its very high boiling point for such a light molecule. At sea level,. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From ceghryvh.blob.core.windows.net
What Is Boiling Point Sea Level at Edwin Acosta blog What Is Responsible For The Relatively High Boiling Point Of Pure Water The most apparent peculiarity of water is its very high boiling point for such a light molecule. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water,. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is Responsible For The Relatively High Boiling Point Of Pure Water At sea level, pure water boils at 100 o c and freezes at o c. The most apparent peculiarity of water is its very high boiling point for such a light molecule. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube What Is Responsible For The Relatively High Boiling Point Of Pure Water At sea level, pure water boils at 100 o c and freezes at o c. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; The. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts What Is Responsible For The Relatively High Boiling Point Of Pure Water Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The “normal” refers to sea level or an elevation of 0 meters or feet. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: At sea level, pure. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is Responsible For The Relatively High Boiling Point Of Pure Water The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; The most. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From chemistrysources.com
diagram مصادر الكيمياء What Is Responsible For The Relatively High Boiling Point Of Pure Water The most apparent peculiarity of water is its very high boiling point for such a light molecule. The “normal” refers to sea level or an elevation of 0 meters or feet. The boiling temperature of water decreases at higher elevations (lower. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. At sea level, pure water. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.youtube.com
2.) Boiling and melting point Grade 7 YouTube What Is Responsible For The Relatively High Boiling Point Of Pure Water Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. At sea level, pure water boils at 100 o c and freezes at o c. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The most apparent. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points Master Organic Chemistry What Is Responsible For The Relatively High Boiling Point Of Pure Water Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The most apparent peculiarity of water is its very high boiling point for such a light molecule. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. At sea level, pure water. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From labonlaptop.com
Find Boiling Point of Water labOnLaptop Store What Is Responsible For The Relatively High Boiling Point Of Pure Water At sea level, pure water boils at 100 o c and freezes at o c. The boiling temperature of water decreases at higher elevations (lower. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The “normal” refers to sea level or an elevation of 0 meters or feet. Hydrogen bonds in. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From imgbin.com
Boiling Point Melting Point Heat Temperature Chemistry PNG, Clipart What Is Responsible For The Relatively High Boiling Point Of Pure Water Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The normal boiling point of water is 100 °c, 212. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.tes.com
Mixtures and Purity GCSE Lesson (SC2a CC2a) Pure or Impure? Teaching What Is Responsible For The Relatively High Boiling Point Of Pure Water The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. The most apparent peculiarity of water is its very high boiling point for such a light molecule. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; Because water seems. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.gauthmath.com
Solved EVAPORATION is the process where a liquid turns into a vapour What Is Responsible For The Relatively High Boiling Point Of Pure Water The “normal” refers to sea level or an elevation of 0 meters or feet. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The most apparent peculiarity of water is its very high boiling point for such a light molecule. The normal boiling point of water is 100 °c, 212 °f,. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From ar.inspiredpencil.com
Boiling Point Of Water Examples What Is Responsible For The Relatively High Boiling Point Of Pure Water Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. At sea level, pure water boils at 100 o c and freezes at o c. The “normal” refers to sea level or an elevation of 0 meters or feet. The normal boiling point of water is. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From askfilo.com
The normal boiling point of pure water is 373.15 K. Calculate the boiling.. What Is Responsible For The Relatively High Boiling Point Of Pure Water At sea level, pure water boils at 100 o c and freezes at o c. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The normal boiling. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From pt.scribd.com
Procedimeito y Pasos para Elaborar Una Misión, Visión, Objetivos, Metas What Is Responsible For The Relatively High Boiling Point Of Pure Water Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; The “normal” refers to sea level or an elevation of 0 meters or feet. The most apparent peculiarity of water is its very high boiling point for such a light molecule. The boiling temperature. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is Responsible For The Relatively High Boiling Point Of Pure Water Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The boiling temperature of water decreases at higher elevations (lower. The “normal” refers to sea level or an elevation of 0 meters or feet. Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point What Is Responsible For The Relatively High Boiling Point Of Pure Water Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From www.researchgate.net
Extraction at boiling point of solvents Download Scientific Diagram What Is Responsible For The Relatively High Boiling Point Of Pure Water The most apparent peculiarity of water is its very high boiling point for such a light molecule. At sea level, pure water boils at 100 o c and freezes at o c. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From gioyxvssk.blob.core.windows.net
What Degrees Is Boiling Point at Charlie Hansel blog What Is Responsible For The Relatively High Boiling Point Of Pure Water Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid; The boiling temperature of water decreases at higher elevations (lower. The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Because water seems so ubiquitous, many people are unaware of. What Is Responsible For The Relatively High Boiling Point Of Pure Water.
From exomkbouq.blob.core.windows.net
What Is Boiling Point Elevation Constant at Ronnie Moore blog What Is Responsible For The Relatively High Boiling Point Of Pure Water Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The normal boiling point of water is 100 °c, 212 °f, or 373.1 k. Hydrogen bonds in water are responsible for values of properties uncommon to a substance comparable to h 2 o, such as h 2 s. Boiling point, temperature at. What Is Responsible For The Relatively High Boiling Point Of Pure Water.