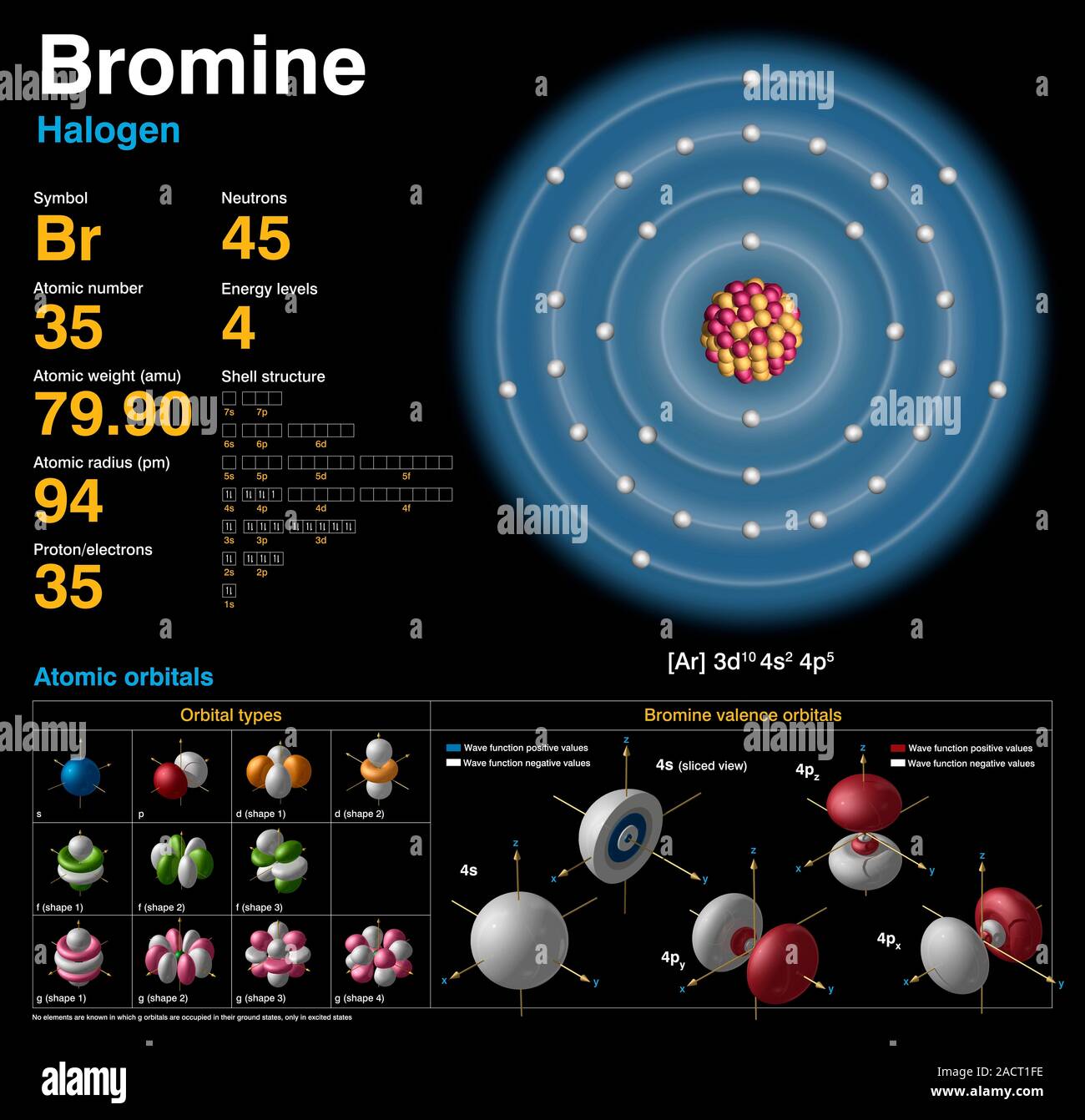
Valence Electrons Of Bromine Atom . The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The first is to use the. The valence electrons are be the 4s and 4p electrons. Bromine has seven valence electrons. There are two ways to find the number of valence electrons in bromine (br). The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). 119 rows valence electrons: Thus, bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower.
from www.alamy.com
The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). The valence electrons are be the 4s and 4p electrons. There are two ways to find the number of valence electrons in bromine (br). The first is to use the. Bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. 119 rows valence electrons: Thus, bromine has seven valence electrons.
Bromine (Br). Diagram of the nuclear composition, electron
Valence Electrons Of Bromine Atom Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Thus, bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. There are two ways to find the number of valence electrons in bromine (br). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. 119 rows valence electrons: The valence electrons are be the 4s and 4p electrons. The first is to use the. Bromine has seven valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵).
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Valence Electrons Of Bromine Atom The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Thus, bromine has seven valence electrons. The valence electrons are be the 4s and 4p electrons. 119 rows valence electrons: 93 rows you may assume. Valence Electrons Of Bromine Atom.
From valenceelectrons.com
Protons, Neutrons, Electrons for Bromine (Br, Br) Valence Electrons Of Bromine Atom Bromine has seven valence electrons. 119 rows valence electrons: Thus, bromine has seven valence electrons. The valence electrons are be the 4s and 4p electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The total number of electrons present in the valence shell of. Valence Electrons Of Bromine Atom.
From brainly.in
bromine valence electrons, Brainly.in Valence Electrons Of Bromine Atom 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Thus, bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The total number of electrons present in the valence. Valence Electrons Of Bromine Atom.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Valence Electrons Of Bromine Atom The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The first is to use. Valence Electrons Of Bromine Atom.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Valence Electrons Of Bromine Atom The valence electrons are be the 4s and 4p electrons. Bromine has seven valence electrons. Thus, bromine has seven valence electrons. 119 rows valence electrons: The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The first is to use the. The total number of electrons present in the. Valence Electrons Of Bromine Atom.
From www.vrogue.co
Symbol And Electron Diagram For Bromine Royalty Free vrogue.co Valence Electrons Of Bromine Atom There are two ways to find the number of valence electrons in bromine (br). Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The valence electrons are be the 4s and 4p electrons. 93 rows you may assume the valences of the chemical. Valence Electrons Of Bromine Atom.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Valence Electrons Of Bromine Atom Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The total number of electrons present in the valence shell of an atom are called valence. Valence Electrons Of Bromine Atom.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Valence Electrons Of Bromine Atom The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). The first is to use the. The valence electrons are be the 4s and 4p electrons. 119 rows valence electrons: Bromine has seven valence electrons.. Valence Electrons Of Bromine Atom.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Valence Electrons Of Bromine Atom There are two ways to find the number of valence electrons in bromine (br). The first is to use the. The valence electrons are be the 4s and 4p electrons. 119 rows valence electrons: Thus, bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond. Valence Electrons Of Bromine Atom.
From mungfali.com
Bromine Electron Dot Diagram Valence Electrons Of Bromine Atom The valence electrons are be the 4s and 4p electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. 119 rows valence electrons: The total. Valence Electrons Of Bromine Atom.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Valence Electrons Of Bromine Atom Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The first is to use the. The valence electrons are be the 4s and. Valence Electrons Of Bromine Atom.
From www.numerade.com
SOLVED For the Bromine atom a) Determine the total number of unpaired Valence Electrons Of Bromine Atom 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. There are two ways to find the number of valence electrons in bromine (br). Bromine has seven valence electrons. 119 rows valence electrons: Thus, bromine has seven valence electrons. The first is to use the. The. Valence Electrons Of Bromine Atom.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Valence Electrons Of Bromine Atom Bromine has seven valence electrons. The valence electrons are be the 4s and 4p electrons. The first is to use the. There are two ways to find the number of valence electrons in bromine (br). The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons. Valence Electrons Of Bromine Atom.
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of Valence Electrons Of Bromine Atom 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Thus, bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The valence electrons are be the 4s and 4p. Valence Electrons Of Bromine Atom.
From www.numerade.com
SOLVED What is the valence electron configuration for the bromine atom? Valence Electrons Of Bromine Atom The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Bromine has seven valence electrons. There are two ways to find the number of valence electrons in bromine (br). 119 rows valence electrons: 93 rows. Valence Electrons Of Bromine Atom.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Valence Electrons Of Bromine Atom The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The total number of electrons present in the valence shell of an atom are called valence electrons, and there. Valence Electrons Of Bromine Atom.
From proper-cooking.info
Bromine Valence Electrons Valence Electrons Of Bromine Atom Bromine has seven valence electrons. Thus, bromine has seven valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). The first is to use the. 93 rows you may assume the valences of. Valence Electrons Of Bromine Atom.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Valence Electrons Of Bromine Atom The valence electrons are be the 4s and 4p electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence. Valence Electrons Of Bromine Atom.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Valence Electrons Of Bromine Atom 119 rows valence electrons: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Bromine has seven valence electrons. The first is to use the. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The. Valence Electrons Of Bromine Atom.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Valence Electrons Of Bromine Atom Thus, bromine has seven valence electrons. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The first is to use the. There are two ways to find the number of valence electrons in bromine (br). The total number of electrons present in the valence shell of an atom are. Valence Electrons Of Bromine Atom.
From www.youtube.com
How many valence electrons does bromine have? YouTube Valence Electrons Of Bromine Atom There are two ways to find the number of valence electrons in bromine (br). The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). The first is to use the. The electron configuration of an. Valence Electrons Of Bromine Atom.
From www.animalia-life.club
Electron Configuration For Bromine Valence Electrons Of Bromine Atom Thus, bromine has seven valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Bromine has seven valence electrons. Only the electrons in the outmost shell are valance electrons.all but seven of the. Valence Electrons Of Bromine Atom.
From valenceelectrons.com
How to Find the Valence Electrons for BrF2 and BrF2+? Valence Electrons Of Bromine Atom Bromine has seven valence electrons. There are two ways to find the number of valence electrons in bromine (br). The first is to use the. Thus, bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. 119 rows valence electrons: Only the electrons in. Valence Electrons Of Bromine Atom.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Valence Electrons Of Bromine Atom The first is to use the. Bromine has seven valence electrons. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. Thus, bromine has seven valence electrons. There are two ways to find the number of valence electrons in bromine (br). The total number of electrons present in the valence. Valence Electrons Of Bromine Atom.
From phootoscelebrities.com
How Many Valence Electrons Does Bromine Have? Valence Electrons Of Bromine Atom The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Bromine has seven valence electrons. The first is to use the. Thus, bromine has seven valence electrons. 93 rows you may assume the valences of. Valence Electrons Of Bromine Atom.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Valence Electrons Of Bromine Atom The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Thus, bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. There are two ways to find the number of. Valence Electrons Of Bromine Atom.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Valence Electrons Of Bromine Atom The first is to use the. Bromine has seven valence electrons. The valence electrons are be the 4s and 4p electrons. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. There are two ways to find the number of valence electrons in bromine (br). 119 rows valence electrons: The. Valence Electrons Of Bromine Atom.
From www.chegg.com
Solved what is the valence electron configuration for the Valence Electrons Of Bromine Atom 119 rows valence electrons: The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Valence Electrons Of Bromine Atom.
From nikitakrystyna.blogspot.com
21+ bromine bohr diagram NikitaKrystyna Valence Electrons Of Bromine Atom Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The valence electrons are be the 4s and 4p electrons. Thus, bromine has seven valence electrons. There are two ways to find the number of valence electrons in bromine (br). 93 rows you may. Valence Electrons Of Bromine Atom.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Valence Electrons Of Bromine Atom The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The first is to use the. Bromine has seven valence electrons. The valence electrons are be the 4s and. Valence Electrons Of Bromine Atom.
From www.slideserve.com
PPT The Parts of an Atom PowerPoint Presentation, free download ID Valence Electrons Of Bromine Atom 119 rows valence electrons: The first is to use the. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Bromine has seven valence electrons. Thus, bromine has seven valence electrons. 93 rows you may. Valence Electrons Of Bromine Atom.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Valence Electrons Of Bromine Atom The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. 119 rows valence electrons: The first is to use the. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence. Valence Electrons Of Bromine Atom.
From valenceelectrons.com
Silver(Ag) electron configuration and orbital diagram Valence Electrons Of Bromine Atom The valence electrons are be the 4s and 4p electrons. 119 rows valence electrons: The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Bromine has seven valence electrons. There are two ways to find the number of valence electrons in bromine (br). Thus, bromine has seven valence electrons.. Valence Electrons Of Bromine Atom.
From proper-cooking.info
Bromine Valence Electrons Valence Electrons Of Bromine Atom 119 rows valence electrons: Thus, bromine has seven valence electrons. Only the electrons in the outmost shell are valance electrons.all but seven of the electrons in bromine are in lower. The valence electrons are be the 4s and 4p electrons. Bromine has seven valence electrons. There are two ways to find the number of valence electrons in bromine (br). 93. Valence Electrons Of Bromine Atom.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Valence Electrons Of Bromine Atom 119 rows valence electrons: Thus, bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present. Valence Electrons Of Bromine Atom.