From brainly.in
when non metal reacts with oxygen it forms non metallic oxide list all What Happens When A Base Reacts With A Non Metallic Oxide For example, the reaction between carbon dioxide and calcium hydroxide. Cl 2 o, so 2, p 4 o 10). You should learn how to construct these equations exactly as. Calcium hydroxide, which is a base,. There is a trend within. Various element oxides can combine with water to produce acids or bases. For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint Presentation, free What Happens When A Base Reacts With A Non Metallic Oxide Various element oxides can combine with water to produce acids or bases. Cl 2 o, so 2, p 4 o 10). For example, 'calcium hydroxide' reacts with. For example, the reaction between carbon dioxide and calcium hydroxide. You should learn how to construct these equations exactly as. Calcium hydroxide, which is a base,. There is a trend within. What Happens When A Base Reacts With A Non Metallic Oxide.
From slidetodoc.com
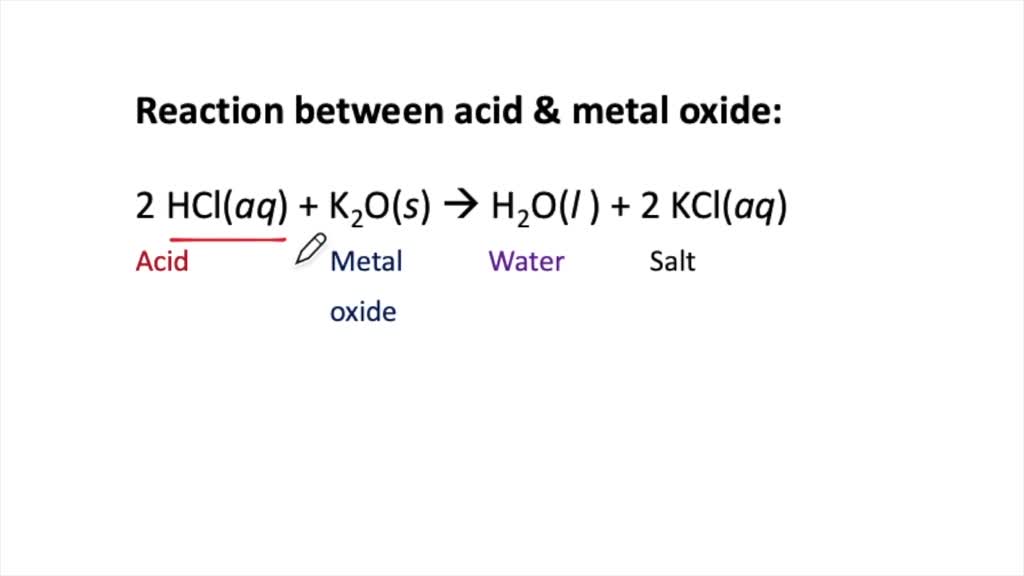
Reaction of Oxides with Acids and Bases Reaction What Happens When A Base Reacts With A Non Metallic Oxide For example, 'calcium hydroxide' reacts with. You should learn how to construct these equations exactly as. Calcium hydroxide, which is a base,. Various element oxides can combine with water to produce acids or bases. Cl 2 o, so 2, p 4 o 10). There is a trend within. For example, the reaction between carbon dioxide and calcium hydroxide. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.youtube.com
How bases react with metals CBSE Class 10 Chemistry Notes YouTube What Happens When A Base Reacts With A Non Metallic Oxide For example, 'calcium hydroxide' reacts with. You should learn how to construct these equations exactly as. For example, the reaction between carbon dioxide and calcium hydroxide. Calcium hydroxide, which is a base,. Cl 2 o, so 2, p 4 o 10). Various element oxides can combine with water to produce acids or bases. There is a trend within. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.teachoo.com
[MCQ] Which of the statements is not correct? All metal oxides react What Happens When A Base Reacts With A Non Metallic Oxide For example, 'calcium hydroxide' reacts with. Cl 2 o, so 2, p 4 o 10). There is a trend within. Calcium hydroxide, which is a base,. For example, the reaction between carbon dioxide and calcium hydroxide. Various element oxides can combine with water to produce acids or bases. You should learn how to construct these equations exactly as. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo What Happens When A Base Reacts With A Non Metallic Oxide For example, 'calcium hydroxide' reacts with. Cl 2 o, so 2, p 4 o 10). Various element oxides can combine with water to produce acids or bases. For example, the reaction between carbon dioxide and calcium hydroxide. You should learn how to construct these equations exactly as. Calcium hydroxide, which is a base,. There is a trend within. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.youtube.com
Nature of metal oxides Metals and Non metals Chemistry Khan What Happens When A Base Reacts With A Non Metallic Oxide There is a trend within. Various element oxides can combine with water to produce acids or bases. For example, the reaction between carbon dioxide and calcium hydroxide. You should learn how to construct these equations exactly as. For example, 'calcium hydroxide' reacts with. Cl 2 o, so 2, p 4 o 10). Calcium hydroxide, which is a base,. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.slideserve.com
PPT 7.5 Types of Reactions & 7.6 Combination Reaction PowerPoint What Happens When A Base Reacts With A Non Metallic Oxide Calcium hydroxide, which is a base,. Cl 2 o, so 2, p 4 o 10). There is a trend within. For example, the reaction between carbon dioxide and calcium hydroxide. For example, 'calcium hydroxide' reacts with. Various element oxides can combine with water to produce acids or bases. You should learn how to construct these equations exactly as. What Happens When A Base Reacts With A Non Metallic Oxide.
From chemistryguru.com.sg
Reaction of Period 3 Oxides with Water, Acids and Bases What Happens When A Base Reacts With A Non Metallic Oxide For example, the reaction between carbon dioxide and calcium hydroxide. For example, 'calcium hydroxide' reacts with. Cl 2 o, so 2, p 4 o 10). Various element oxides can combine with water to produce acids or bases. You should learn how to construct these equations exactly as. Calcium hydroxide, which is a base,. There is a trend within. What Happens When A Base Reacts With A Non Metallic Oxide.
From byjus.com
base + metal ? base + non metal oxide What Happens When A Base Reacts With A Non Metallic Oxide Various element oxides can combine with water to produce acids or bases. There is a trend within. Calcium hydroxide, which is a base,. For example, 'calcium hydroxide' reacts with. Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. You should learn how to construct these equations exactly as. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.youtube.com
Chemical Properties of acid with metallic Oxides and Metal carbonate What Happens When A Base Reacts With A Non Metallic Oxide There is a trend within. You should learn how to construct these equations exactly as. Various element oxides can combine with water to produce acids or bases. Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. For example, 'calcium hydroxide' reacts with. Calcium hydroxide, which is a base,. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.youtube.com
How acids react with metallic oxides CBSE Class 10 Chemistry Notes What Happens When A Base Reacts With A Non Metallic Oxide There is a trend within. For example, 'calcium hydroxide' reacts with. You should learn how to construct these equations exactly as. Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. Various element oxides can combine with water to produce acids or bases. Calcium hydroxide, which is a base,. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts What Happens When A Base Reacts With A Non Metallic Oxide Calcium hydroxide, which is a base,. Various element oxides can combine with water to produce acids or bases. You should learn how to construct these equations exactly as. For example, 'calcium hydroxide' reacts with. For example, the reaction between carbon dioxide and calcium hydroxide. There is a trend within. Cl 2 o, so 2, p 4 o 10). What Happens When A Base Reacts With A Non Metallic Oxide.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts What Happens When A Base Reacts With A Non Metallic Oxide There is a trend within. For example, the reaction between carbon dioxide and calcium hydroxide. Calcium hydroxide, which is a base,. You should learn how to construct these equations exactly as. Cl 2 o, so 2, p 4 o 10). Various element oxides can combine with water to produce acids or bases. For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.nagwa.com
Question Video Identifying the Type of Substance Formed When a What Happens When A Base Reacts With A Non Metallic Oxide You should learn how to construct these equations exactly as. For example, the reaction between carbon dioxide and calcium hydroxide. There is a trend within. Calcium hydroxide, which is a base,. Various element oxides can combine with water to produce acids or bases. Cl 2 o, so 2, p 4 o 10). For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From slideplayer.com
compounds. ppt download What Happens When A Base Reacts With A Non Metallic Oxide Cl 2 o, so 2, p 4 o 10). Calcium hydroxide, which is a base,. You should learn how to construct these equations exactly as. There is a trend within. For example, the reaction between carbon dioxide and calcium hydroxide. Various element oxides can combine with water to produce acids or bases. For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From askfilo.com
(nonmetal) reacts with base [Ca(OH)2 ] and forms salt and water. What do.. What Happens When A Base Reacts With A Non Metallic Oxide Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. For example, 'calcium hydroxide' reacts with. Calcium hydroxide, which is a base,. You should learn how to construct these equations exactly as. There is a trend within. Various element oxides can combine with water to produce acids or bases. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.youtube.com
Reaction Of bases With NonMetal Oxides 10 YouTube What Happens When A Base Reacts With A Non Metallic Oxide For example, 'calcium hydroxide' reacts with. Calcium hydroxide, which is a base,. Various element oxides can combine with water to produce acids or bases. There is a trend within. You should learn how to construct these equations exactly as. For example, the reaction between carbon dioxide and calcium hydroxide. Cl 2 o, so 2, p 4 o 10). What Happens When A Base Reacts With A Non Metallic Oxide.
From slidetodoc.com
Reaction of Oxides with Acids and Bases Reaction What Happens When A Base Reacts With A Non Metallic Oxide Calcium hydroxide, which is a base,. There is a trend within. Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. Various element oxides can combine with water to produce acids or bases. You should learn how to construct these equations exactly as. For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.youtube.com
Reaction of Metallic oxides YouTube What Happens When A Base Reacts With A Non Metallic Oxide Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. For example, 'calcium hydroxide' reacts with. Calcium hydroxide, which is a base,. Various element oxides can combine with water to produce acids or bases. There is a trend within. You should learn how to construct these equations exactly as. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.numerade.com
SOLVEDList an example of a reaction between an acid and a metal oxide. What Happens When A Base Reacts With A Non Metallic Oxide Calcium hydroxide, which is a base,. There is a trend within. Various element oxides can combine with water to produce acids or bases. You should learn how to construct these equations exactly as. For example, the reaction between carbon dioxide and calcium hydroxide. Cl 2 o, so 2, p 4 o 10). For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.slideshare.net
Oxides What Happens When A Base Reacts With A Non Metallic Oxide Cl 2 o, so 2, p 4 o 10). Various element oxides can combine with water to produce acids or bases. There is a trend within. You should learn how to construct these equations exactly as. Calcium hydroxide, which is a base,. For example, the reaction between carbon dioxide and calcium hydroxide. For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.teachoo.com
Reaction of Metals and Nonmetals With Base Teachoo Concepts What Happens When A Base Reacts With A Non Metallic Oxide There is a trend within. For example, 'calcium hydroxide' reacts with. You should learn how to construct these equations exactly as. Various element oxides can combine with water to produce acids or bases. Calcium hydroxide, which is a base,. Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.teachoo.com
What type of oxides are formed when nonmetals combine with oxygen? What Happens When A Base Reacts With A Non Metallic Oxide There is a trend within. Calcium hydroxide, which is a base,. For example, the reaction between carbon dioxide and calcium hydroxide. Cl 2 o, so 2, p 4 o 10). You should learn how to construct these equations exactly as. Various element oxides can combine with water to produce acids or bases. For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From slidetodoc.com
Reaction of Oxides with Acids and Bases Reaction What Happens When A Base Reacts With A Non Metallic Oxide You should learn how to construct these equations exactly as. Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. There is a trend within. For example, 'calcium hydroxide' reacts with. Various element oxides can combine with water to produce acids or bases. Calcium hydroxide, which is a base,. What Happens When A Base Reacts With A Non Metallic Oxide.
From byjus.com
What are the COMMON NATURES and SIMILARITIES of Metal Oxides and Non What Happens When A Base Reacts With A Non Metallic Oxide There is a trend within. Various element oxides can combine with water to produce acids or bases. You should learn how to construct these equations exactly as. For example, the reaction between carbon dioxide and calcium hydroxide. Cl 2 o, so 2, p 4 o 10). Calcium hydroxide, which is a base,. For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.numerade.com
Metal oxides can react with water to form bases. What Happens When A Base Reacts With A Non Metallic Oxide Calcium hydroxide, which is a base,. For example, the reaction between carbon dioxide and calcium hydroxide. Various element oxides can combine with water to produce acids or bases. For example, 'calcium hydroxide' reacts with. You should learn how to construct these equations exactly as. Cl 2 o, so 2, p 4 o 10). There is a trend within. What Happens When A Base Reacts With A Non Metallic Oxide.
From ar.inspiredpencil.com
Metal And Water Reaction What Happens When A Base Reacts With A Non Metallic Oxide For example, the reaction between carbon dioxide and calcium hydroxide. Calcium hydroxide, which is a base,. For example, 'calcium hydroxide' reacts with. You should learn how to construct these equations exactly as. Various element oxides can combine with water to produce acids or bases. Cl 2 o, so 2, p 4 o 10). There is a trend within. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID333264 What Happens When A Base Reacts With A Non Metallic Oxide Various element oxides can combine with water to produce acids or bases. There is a trend within. For example, the reaction between carbon dioxide and calcium hydroxide. Calcium hydroxide, which is a base,. For example, 'calcium hydroxide' reacts with. You should learn how to construct these equations exactly as. Cl 2 o, so 2, p 4 o 10). What Happens When A Base Reacts With A Non Metallic Oxide.
From www.youtube.com
AE6 Non metal oxides YouTube What Happens When A Base Reacts With A Non Metallic Oxide Cl 2 o, so 2, p 4 o 10). For example, 'calcium hydroxide' reacts with. Calcium hydroxide, which is a base,. For example, the reaction between carbon dioxide and calcium hydroxide. You should learn how to construct these equations exactly as. Various element oxides can combine with water to produce acids or bases. There is a trend within. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with What Happens When A Base Reacts With A Non Metallic Oxide Cl 2 o, so 2, p 4 o 10). You should learn how to construct these equations exactly as. There is a trend within. Various element oxides can combine with water to produce acids or bases. For example, 'calcium hydroxide' reacts with. For example, the reaction between carbon dioxide and calcium hydroxide. Calcium hydroxide, which is a base,. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free What Happens When A Base Reacts With A Non Metallic Oxide For example, 'calcium hydroxide' reacts with. For example, the reaction between carbon dioxide and calcium hydroxide. Calcium hydroxide, which is a base,. You should learn how to construct these equations exactly as. Cl 2 o, so 2, p 4 o 10). There is a trend within. Various element oxides can combine with water to produce acids or bases. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.youtube.com
Acids Bases and Salts 6 Metal and nonmetal oxides reaction with What Happens When A Base Reacts With A Non Metallic Oxide For example, 'calcium hydroxide' reacts with. Calcium hydroxide, which is a base,. Various element oxides can combine with water to produce acids or bases. There is a trend within. You should learn how to construct these equations exactly as. Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.teachoo.com
Sample Paper (MCQ) The table given below shows the reaction of a few What Happens When A Base Reacts With A Non Metallic Oxide Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. You should learn how to construct these equations exactly as. There is a trend within. Various element oxides can combine with water to produce acids or bases. Calcium hydroxide, which is a base,. For example, 'calcium hydroxide' reacts with. What Happens When A Base Reacts With A Non Metallic Oxide.
From www.youtube.com
How bases react with non metallic oxides CBSE Class 10 Chemistry Notes What Happens When A Base Reacts With A Non Metallic Oxide You should learn how to construct these equations exactly as. Cl 2 o, so 2, p 4 o 10). For example, the reaction between carbon dioxide and calcium hydroxide. For example, 'calcium hydroxide' reacts with. There is a trend within. Various element oxides can combine with water to produce acids or bases. Calcium hydroxide, which is a base,. What Happens When A Base Reacts With A Non Metallic Oxide.