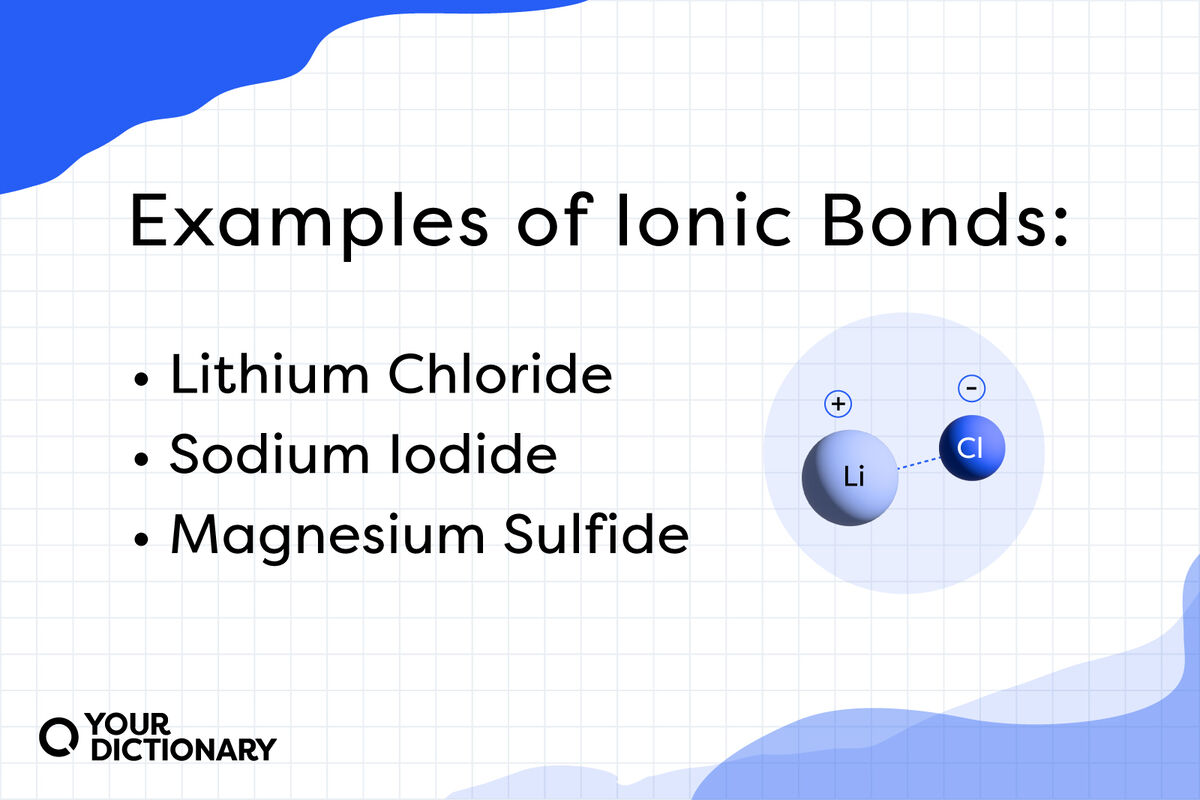
Ionic Compounds Description . ionic compounds are composed of cations and anions that are strongly attracted to each other. ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. Because of the polarity of the chemical bond, many ionic compounds dissolve in water. Table salt, nacl, is an ionic compound. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. Hence, ionic solids have very. An ionic compound is a compound formed by ions bonding together through electrostatic forces. Another example is silver iodide, agi. ionic compound definition:
from ar.inspiredpencil.com
Table salt, nacl, is an ionic compound. Another example is silver iodide, agi. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. ionic compound definition: ionic compounds are composed of cations and anions that are strongly attracted to each other. Hence, ionic solids have very. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An ionic compound is a compound formed by ions bonding together through electrostatic forces.
Ionic Compound List
Ionic Compounds Description Table salt, nacl, is an ionic compound. ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. Another example is silver iodide, agi. Table salt, nacl, is an ionic compound. Because of the polarity of the chemical bond, many ionic compounds dissolve in water. ionic compound definition: ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ionic compounds are composed of cations and anions that are strongly attracted to each other. Hence, ionic solids have very. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. An ionic compound is a compound formed by ions bonding together through electrostatic forces.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID2682442 Ionic Compounds Description ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. An ionic compound is a compound formed by ions bonding together through electrostatic forces. Because of the polarity of the chemical bond, many ionic compounds dissolve in water. Table salt, nacl, is an ionic compound. Ionic compounds have high. Ionic Compounds Description.
From www.animalia-life.club
Ionic Compounds Periodic Table Ionic Compounds Description ionic compounds are composed of cations and anions that are strongly attracted to each other. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. An ionic compound is. Ionic Compounds Description.
From www.slideserve.com
PPT Properties of Ionic Compounds PowerPoint Presentation, free Ionic Compounds Description ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of. Ionic Compounds Description.
From studylib.net
Naming Ionic Compounds Ionic Compounds Description ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Another example is silver iodide, agi. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. ionic compound definition: Ionic compounds have high melting and boiling. Ionic Compounds Description.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Ionic Compounds Description ionic compounds are composed of cations and anions that are strongly attracted to each other. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. An ionic compound is a compound formed by ions bonding together through electrostatic forces. ionic bonds produce compound that conduct electricity when dissolved or molten. Ionic Compounds Description.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Ionic Compounds Description ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ionic compound definition: Hence, ionic solids have very. Because of the polarity of the chemical bond,. Ionic Compounds Description.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Ionic Compounds Description ionic compounds are composed of cations and anions that are strongly attracted to each other. ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. Another example is silver iodide, agi. Ionic compounds have high melting and boiling points, so they are in the solid state at. Ionic Compounds Description.
From en.wikipedia.org
Ionic bonding Wikipedia Ionic Compounds Description ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. An ionic compound is a compound formed by ions bonding together through electrostatic forces. Hence, ionic solids have very. ionic compound definition: Because of the polarity of the chemical bond, many ionic compounds dissolve in water. Ionic. Ionic Compounds Description.
From www.learnatnoon.com
Physical Properties of an Ionic Compound Noon Academy Ionic Compounds Description Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. Another example is silver iodide, agi. Because of the polarity of the chemical bond, many ionic compounds dissolve in water. An. Ionic Compounds Description.
From scientifictutor.org
Chem Naming Ionic Compounds Part 1 Scientific Tutor Ionic Compounds Description Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Ionic Compounds Description.
From www.youtube.com
Properties of ionic compounds YouTube Ionic Compounds Description ionic compound definition: ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compounds are composed of cations and anions that are strongly attracted to each other.. Ionic Compounds Description.
From www.slideserve.com
PPT CHAPTER 3 Ionic Compounds General, Organic, & Biological Ionic Compounds Description Another example is silver iodide, agi. ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. Table salt, nacl, is an ionic compound. An. Ionic Compounds Description.
From studylib.net
Ionic Compounds Naming Ionic Compounds Description ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. Because of the polarity of the chemical bond, many ionic compounds dissolve in water. An ionic compound is a compound formed by ions bonding together through electrostatic forces. Table salt, nacl, is an ionic compound. ionic compound, any. Ionic Compounds Description.
From www.scribd.com
Ion Chemical Compounds Ionic Compounds Description ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ionic compounds are composed of cations and anions that are strongly attracted to each other. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. An ionic compound is. Ionic Compounds Description.
From www.breakingatom.com
Ionic Properties Ionic Compounds Description Another example is silver iodide, agi. Table salt, nacl, is an ionic compound. Because of the polarity of the chemical bond, many ionic compounds dissolve in water. ionic compounds are composed of cations and anions that are strongly attracted to each other. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein. Ionic Compounds Description.
From www.pinterest.com
3.5 Ionic Compounds Formulas and Names Teaching chemistry, Chemistry Ionic Compounds Description ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Ionic Compounds Description.
From www.vrogue.co
What Are Ionic Compounds Definition Examples Reaction vrogue.co Ionic Compounds Description An ionic compound is a compound formed by ions bonding together through electrostatic forces. ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. Ionic. Ionic Compounds Description.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Ionic Compounds Description ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ionic compound definition: An ionic compound is a compound formed by ions bonding together through electrostatic forces. Because of the polarity of the chemical bond, many ionic compounds dissolve in water. Hence, ionic solids have very. ionic compound, any. Ionic Compounds Description.
From www.pinterest.com
Properties of Ionic Compounds Ionic compound, Ionic, Chemistry lessons Ionic Compounds Description ionic compounds are composed of cations and anions that are strongly attracted to each other. Hence, ionic solids have very. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Another example is silver iodide, agi. ionic compounds are good conductors of electricity in their molten or fused state due. Ionic Compounds Description.
From saylordotorg.github.io
Naming Ionic Compounds Ionic Compounds Description Another example is silver iodide, agi. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compound definition: Table salt, nacl, is an ionic compound. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. ionic compounds are. Ionic Compounds Description.
From quizzcampusparis123.z21.web.core.windows.net
Nomenclature Worksheet 3 Ionic Compounds Containing Polyatom Ionic Compounds Description Table salt, nacl, is an ionic compound. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. Another example is silver iodide, agi. Hence, ionic solids have very. ionic compounds are composed of cations and anions that are strongly attracted to each other. ionic bonds produce compound. Ionic Compounds Description.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Ionic Compounds Description ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compounds are composed of cations and anions that are strongly attracted to each other. ionic. Ionic Compounds Description.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Ionic Compounds Description Because of the polarity of the chemical bond, many ionic compounds dissolve in water. ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. An ionic compound. Ionic Compounds Description.
From sciencenotes.org
Examples of Ionic Compounds in Everyday Life Ionic Compounds Description ionic compound definition: Table salt, nacl, is an ionic compound. Because of the polarity of the chemical bond, many ionic compounds dissolve in water. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Another example is silver iodide, agi. ionic compounds are composed of cations and anions that are. Ionic Compounds Description.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Ionic Compounds Description ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. Table salt, nacl, is an ionic compound. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compound definition: An ionic compound is a compound formed by ions. Ionic Compounds Description.
From testbook.com
Formation Of Ionic Compounds Learn Definition, Examples & Uses Ionic Compounds Description Because of the polarity of the chemical bond, many ionic compounds dissolve in water. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ionic compounds are composed of cations and anions that are strongly attracted to each other. An ionic compound is a compound formed by ions bonding together. Ionic Compounds Description.
From ar.inspiredpencil.com
Ionic Compound List Ionic Compounds Description ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. Ionic compounds have high melting and boiling points, so they are in the solid state at room. Ionic Compounds Description.
From ar.inspiredpencil.com
Ionic Compound List Ionic Compounds Description An ionic compound is a compound formed by ions bonding together through electrostatic forces. ionic compound definition: ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. ionic compounds are good conductors of electricity in their molten or fused state due to the presence. Ionic Compounds Description.
From quizizz.com
Properties of ionic compounds Chemistry Quizizz Ionic Compounds Description ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Hence, ionic. Ionic Compounds Description.
From www.slideserve.com
PPT NAMING IONIC COMPOUNDS PowerPoint Presentation, free download Ionic Compounds Description Table salt, nacl, is an ionic compound. ionic compound definition: ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Hence, ionic solids have very. ionic compound, any of a large. Ionic Compounds Description.
From www.madebyteachers.com
Naming and Formulas of Ionic Compounds A Chemistry Worksheet Made By Ionic Compounds Description ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. ionic compound definition: ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. ionic bond, type of linkage formed from the electrostatic attraction between oppositely. Ionic Compounds Description.
From studylib.net
Naming Ionic Compounds Flow Chart Ionic Compounds Description Because of the polarity of the chemical bond, many ionic compounds dissolve in water. ionic bonds produce compound that conduct electricity when dissolved or molten and generally have high melting and boiling points as solids. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. ionic compounds are good. Ionic Compounds Description.
From www.studypool.com
SOLUTION Chemical formula and naming of ionic compound Studypool Ionic Compounds Description Because of the polarity of the chemical bond, many ionic compounds dissolve in water. Hence, ionic solids have very. Table salt, nacl, is an ionic compound. ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. ionic compound definition: Ionic compounds have high melting and boiling points, so. Ionic Compounds Description.
From www.slideserve.com
PPT Ionic Compounds Names and Formulas PowerPoint Presentation, free Ionic Compounds Description ionic compounds are good conductors of electricity in their molten or fused state due to the presence of free ions. Because of the polarity of the chemical bond, many ionic compounds dissolve in water. ionic compounds are composed of cations and anions that are strongly attracted to each other. ionic bond, type of linkage formed from the. Ionic Compounds Description.
From spmchemistry.blog.onlinetuition.com.my
Physical Properties Ionic Compounds SPM Chemistry Ionic Compounds Description ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron transfer, or ionic bonding, holds the. ionic compound definition: Because of the polarity of the chemical bond, many ionic compounds dissolve in water. ionic compounds are composed of cations and anions that are strongly attracted to each other. An ionic. Ionic Compounds Description.