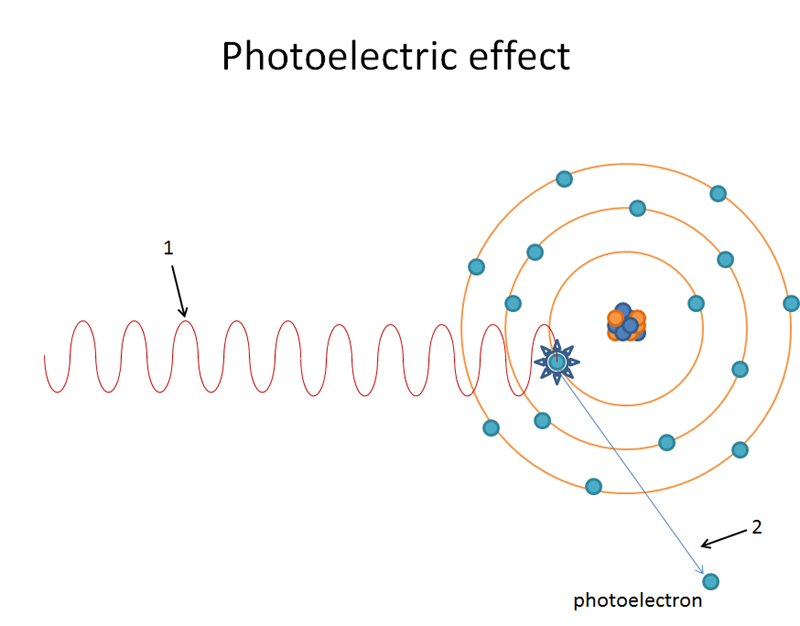
Is It Always Possible To See Photoelectric Effect With Red Light . See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. If you shine high energy light on some atoms, electrons will be blasted off the. The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. The photoelectric effect is the name for this phenomenon: When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right?
from effectinforme.blogspot.com
If you shine high energy light on some atoms, electrons will be blasted off the. The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. The photoelectric effect is the name for this phenomenon: So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right?
Photoelectric effect Effect Information Center
Is It Always Possible To See Photoelectric Effect With Red Light So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. If you shine high energy light on some atoms, electrons will be blasted off the. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? The photoelectric effect is the name for this phenomenon: When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum.
From bumens.weebly.com
Light intensity photoelectric effect equation bumens Is It Always Possible To See Photoelectric Effect With Red Light When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. If you shine high energy light on some atoms, electrons will be blasted. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.slideshare.net
Photoelectric effect ppt Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect is the name for this phenomenon: See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up. Is It Always Possible To See Photoelectric Effect With Red Light.
From sci.esa.int
ESA Science & Technology The photoelectric effect Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect is the name for this phenomenon: The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. When a metal surface is exposed to a. Is It Always Possible To See Photoelectric Effect With Red Light.
From ar.inspiredpencil.com
Photoelectric Effect Is It Always Possible To See Photoelectric Effect With Red Light See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. If you shine high energy light on some atoms, electrons will be blasted off the. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.mdpi.com
Crystals Free FullText LED as Transmitter and Receiver of Light A Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect is the name for this phenomenon: So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? The photoelectric effect does not occur when the red light strikes the metallic surface. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.slideserve.com
PPT Photoelectric effect, photons PowerPoint Presentation, free Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. If you shine high energy light on some atoms, electrons will be blasted off the. So in order to see the same effect with red light as we saw with blue light, we just need to. Is It Always Possible To See Photoelectric Effect With Red Light.
From scottbembenek.com
The Photoelectric Effect Scott D. Bembenek Is It Always Possible To See Photoelectric Effect With Red Light If you shine high energy light on some atoms, electrons will be blasted off the. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? When a metal surface is exposed to a. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.scienceabc.com
What Is The Photoelectric Effect? » ScienceABC Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect is the name for this phenomenon: When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. So in order to see the same effect with. Is It Always Possible To See Photoelectric Effect With Red Light.
From general.chemistrysteps.com
Photoelectric Effect Chemistry Steps Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. If you shine high energy light on some atoms, electrons will be blasted off the. So in order to see the same effect with red light as we saw with blue light, we just need to. Is It Always Possible To See Photoelectric Effect With Red Light.
From partmcveighkathakalis.z21.web.core.windows.net
Photoelectric Effect Diagram Is It Always Possible To See Photoelectric Effect With Red Light See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. The photoelectric effect is the name for this phenomenon: If you shine high energy light on some atoms, electrons will be blasted off the. The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency. Is It Always Possible To See Photoelectric Effect With Red Light.
From myfreeslides.com
Free Photoelectric Effect Presentation Template MyFreeSlides Is It Always Possible To See Photoelectric Effect With Red Light If you shine high energy light on some atoms, electrons will be blasted off the. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. So in order to see the same effect with red light as we saw with blue light, we just need to crank. Is It Always Possible To See Photoelectric Effect With Red Light.
From syzygyastro.hubpages.com
How the Physics of can Generate Electricity HubPages Is It Always Possible To See Photoelectric Effect With Red Light So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. The photoelectric effect does. Is It Always Possible To See Photoelectric Effect With Red Light.
From schematiclistpact101.z22.web.core.windows.net
Explain Photoelectric Effect With Diagram Is It Always Possible To See Photoelectric Effect With Red Light When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right?. Is It Always Possible To See Photoelectric Effect With Red Light.
From slideplayer.com
Photoelectric effect, photons ppt download Is It Always Possible To See Photoelectric Effect With Red Light So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident.. Is It Always Possible To See Photoelectric Effect With Red Light.
From notes.thatother.dev
Photoelectric Effect Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. So in order to see the same effect with red light as we. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.youtube.com
The Photoelectric Effect and How it Works YouTube Is It Always Possible To See Photoelectric Effect With Red Light So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident.. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.youtube.com
The Photoelectric Effect YouTube Is It Always Possible To See Photoelectric Effect With Red Light When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right?. Is It Always Possible To See Photoelectric Effect With Red Light.
From effectinforme.blogspot.com
Photoelectric effect Effect Information Center Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect is the name for this phenomenon: When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. The photoelectric effect does not occur when the red. Is It Always Possible To See Photoelectric Effect With Red Light.
From quizizz.com
The photoelectric effect Quizizz Is It Always Possible To See Photoelectric Effect With Red Light See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? If you shine high. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.nsta.org
The Photoelectric Effect NSTA Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect is the name for this phenomenon: When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red. Is It Always Possible To See Photoelectric Effect With Red Light.
From general.chemistrysteps.com
Photoelectric Effect Chemistry Steps Is It Always Possible To See Photoelectric Effect With Red Light See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. The photoelectric effect is the name for this phenomenon: When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. So in order to see the same effect with. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.scienceabc.com
What Is The Photoelectric Effect? » ScienceABC Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency,. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.physicstutoronline.co.uk
Photoelectric Effect explained in this fully illustrated article Is It Always Possible To See Photoelectric Effect With Red Light So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. When a metal surface. Is It Always Possible To See Photoelectric Effect With Red Light.
From byjus.com
Einstein's Explanation Of Photoelectric Effect Threshold Frequency Is It Always Possible To See Photoelectric Effect With Red Light When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. The photoelectric effect is the name for this phenomenon: So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red. Is It Always Possible To See Photoelectric Effect With Red Light.
From fyopqwpba.blob.core.windows.net
Photoelectric Experiment Explained at Linda Villarreal blog Is It Always Possible To See Photoelectric Effect With Red Light When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. So in order to see the same effect with red light as we. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.livescience.com
Photoelectric Effect Explanation & Applications Live Science Is It Always Possible To See Photoelectric Effect With Red Light So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? The photoelectric effect is the name for this phenomenon: See how light knocks electrons off a metal target, and recreate the experiment that. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.nsta.org
The Photoelectric Effect NSTA Is It Always Possible To See Photoelectric Effect With Red Light So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. If you shine high. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.nuclear-power.com
Definition of Photoelectric Effect Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. The photoelectric effect is the name for this phenomenon: If you shine high energy light on some atoms, electrons will be blasted off the. See how light knocks electrons off a metal target, and recreate the. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.pinterest.com
Photoelectric Effect Easy Science Easy science, Stem for kids Is It Always Possible To See Photoelectric Effect With Red Light If you shine high energy light on some atoms, electrons will be blasted off the. The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. The photoelectric effect is the name for this phenomenon: So in order to see the same effect with red light as. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.slideserve.com
PPT Photoelectric Effect PowerPoint Presentation, free download ID Is It Always Possible To See Photoelectric Effect With Red Light See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. So in order to see the same effect with red light as we saw with blue light, we. Is It Always Possible To See Photoelectric Effect With Red Light.
From effectinforme.blogspot.com
Photoelectric effect Effect Information Center Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect does not occur when the red light strikes the metallic surface because the frequency of red light is lower than the. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. See how light knocks electrons off a metal target, and recreate the experiment. Is It Always Possible To See Photoelectric Effect With Red Light.
From lubbil.com
The Photoelectric Effect (2023) Is It Always Possible To See Photoelectric Effect With Red Light See how light knocks electrons off a metal target, and recreate the experiment that spawned the field of quantum. The photoelectric effect is the name for this phenomenon: So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.slideshare.net
Photoelectric effect Is It Always Possible To See Photoelectric Effect With Red Light So in order to see the same effect with red light as we saw with blue light, we just need to crank up the intensity of the red light to make up for the energy deficiency, right? If you shine high energy light on some atoms, electrons will be blasted off the. The photoelectric effect does not occur when the. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.physicstutoronline.co.uk
Photoelectric Effect explained in this fully illustrated article Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect is the name for this phenomenon: If you shine high energy light on some atoms, electrons will be blasted off the. When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. See how light knocks electrons off a metal target, and recreate the experiment. Is It Always Possible To See Photoelectric Effect With Red Light.
From www.physicstutoronline.co.uk
Photoelectric Effect explained in this fully illustrated article Is It Always Possible To See Photoelectric Effect With Red Light The photoelectric effect is the name for this phenomenon: When a metal surface is exposed to a monochromatic electromagnetic wave of sufficiently short wavelength (or equivalently, above a threshold frequency), the incident. If you shine high energy light on some atoms, electrons will be blasted off the. So in order to see the same effect with red light as we. Is It Always Possible To See Photoelectric Effect With Red Light.