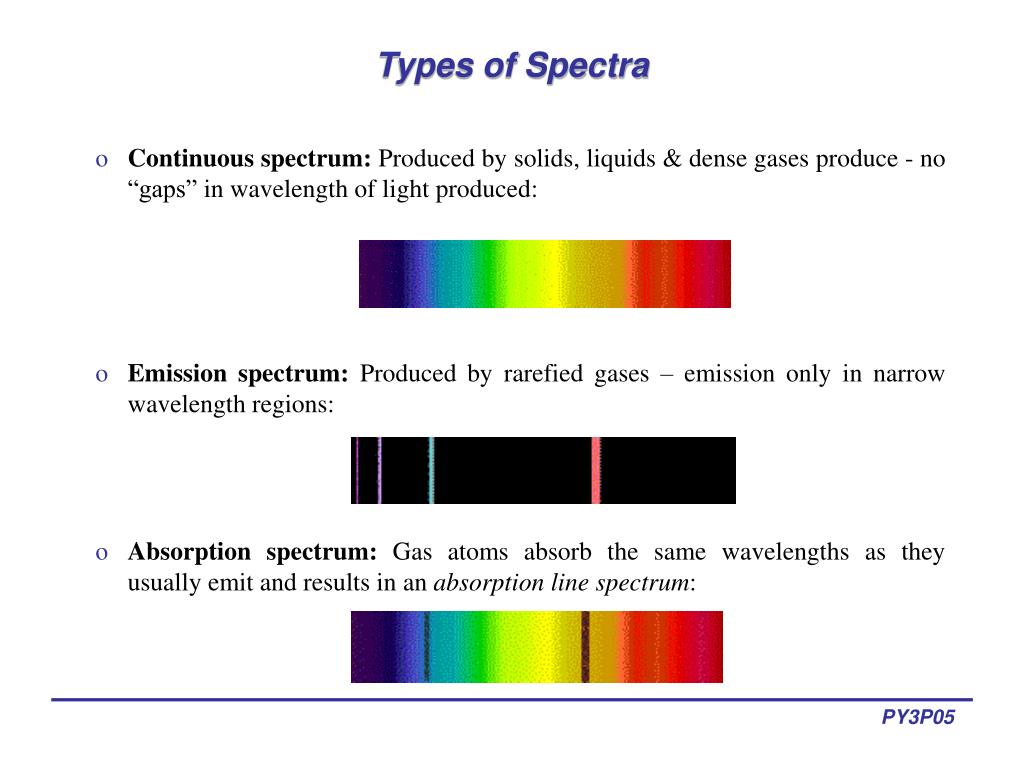
Spectroscopy A Virtual Lab Element Identification And Emission Spectra . When an atom or ion in substance got excited, they emit radiations of particular frequencies. Emission line spectra for selected elements) to observe the emission spectra for known. Use the virtual spectroscopy lab (part 2: Spectroscope you can view the emission line spectra. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. 2.use a flame test to observe the color produced when metal ions. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. 1.observe the bright line spectra (emission spectra) for various elements. Different elements produce different spectra that are unique enough to be. These radiations are seen in the form of.
from www.slideserve.com
The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. When an atom or ion in substance got excited, they emit radiations of particular frequencies. Emission line spectra for selected elements) to observe the emission spectra for known. 1.observe the bright line spectra (emission spectra) for various elements. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. Use the virtual spectroscopy lab (part 2: When hydrogen gas is placed. Spectroscope you can view the emission line spectra. 2.use a flame test to observe the color produced when metal ions. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint
Spectroscopy A Virtual Lab Element Identification And Emission Spectra Emission line spectra for selected elements) to observe the emission spectra for known. 2.use a flame test to observe the color produced when metal ions. These radiations are seen in the form of. 1.observe the bright line spectra (emission spectra) for various elements. Different elements produce different spectra that are unique enough to be. Spectroscope you can view the emission line spectra. When hydrogen gas is placed. Use the virtual spectroscopy lab (part 2: When an atom or ion in substance got excited, they emit radiations of particular frequencies. The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission line spectra for selected elements) to observe the emission spectra for known.
From mungfali.com
Emission Spectrum Of All Elements Spectroscopy A Virtual Lab Element Identification And Emission Spectra The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 1.observe the bright line spectra (emission spectra) for various elements. When an atom or ion in substance got excited, they emit. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.slideserve.com
PPT Flame Spectroscopy Lab PowerPoint Presentation, free download Spectroscopy A Virtual Lab Element Identification And Emission Spectra Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. These radiations are seen in the form of. 1.observe the bright line spectra (emission spectra) for various elements. Emission line spectra for selected elements) to observe the emission spectra for known. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From studylib.net
Spectroscopy A Virtual Lab Spectroscopy A Virtual Lab Element Identification And Emission Spectra A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission line spectra for selected elements) to observe the emission. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From physicsopenlab.org
Arc Atomic Emission Spectroscopy PhysicsOpenLab Spectroscopy A Virtual Lab Element Identification And Emission Spectra 1.observe the bright line spectra (emission spectra) for various elements. These radiations are seen in the form of. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission line spectra for selected elements) to observe the emission spectra for known. A virtual lab. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Spectroscopy A Virtual Lab Element Identification And Emission Spectra Use the virtual spectroscopy lab (part 2: The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. Spectroscope you can view the emission line spectra.. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From ar.inspiredpencil.com
Spectrum Noble Gases Spectroscopy A Virtual Lab Element Identification And Emission Spectra When an atom or ion in substance got excited, they emit radiations of particular frequencies. These radiations are seen in the form of. Emission line spectra for selected elements) to observe the emission spectra for known. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Use the virtual spectroscopy lab (part 2: The unique color that. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Spectroscopy A Virtual Lab Element Identification And Emission Spectra When an atom or ion in substance got excited, they emit radiations of particular frequencies. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From studylib.net
Lab 7 Using Emission Spectra to Identify Pure Element Spectroscopy A Virtual Lab Element Identification And Emission Spectra These radiations are seen in the form of. When hydrogen gas is placed. Spectroscope you can view the emission line spectra. 1.observe the bright line spectra (emission spectra) for various elements. 2.use a flame test to observe the color produced when metal ions. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectroscopy A Virtual Lab Element Identification And Emission Spectra When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. These radiations are seen in. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From courses.lumenlearning.com
Spectroscopy in Astronomy Astronomy Spectroscopy A Virtual Lab Element Identification And Emission Spectra Use the virtual spectroscopy lab (part 2: Different elements produce different spectra that are unique enough to be. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. Spectroscope you can view the emission line spectra. These radiations are seen in the form of. The emission. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From quizzfullmarks77.z19.web.core.windows.net
Atomic Emission Spectra Lab Pdf Spectroscopy A Virtual Lab Element Identification And Emission Spectra 2.use a flame test to observe the color produced when metal ions. The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. Spectroscope you can view the emission line spectra. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.savemyexams.com
Flame Emission Spectroscopy AQA GCSE Chemistry Revision Notes 2018 Spectroscopy A Virtual Lab Element Identification And Emission Spectra 1.observe the bright line spectra (emission spectra) for various elements. Use the virtual spectroscopy lab (part 2: When hydrogen gas is placed. Different elements produce different spectra that are unique enough to be. Spectroscope you can view the emission line spectra. Emission line spectra for selected elements) to observe the emission spectra for known. The emission spectrum (or line spectrum). Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From studylib.net
Every element has its own characteristic spectral emission Spectroscopy A Virtual Lab Element Identification And Emission Spectra Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light.. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectroscopy A Virtual Lab Element Identification And Emission Spectra These radiations are seen in the form of. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Build and. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From spiff.rit.edu
Spectrographs and Spectra Spectroscopy A Virtual Lab Element Identification And Emission Spectra These radiations are seen in the form of. Use the virtual spectroscopy lab (part 2: A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. Spectroscope you can view the emission line spectra. 1.observe the bright line spectra (emission spectra) for various elements. When hydrogen gas. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Spectroscopy A Virtual Lab Element Identification And Emission Spectra Use the virtual spectroscopy lab (part 2: A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. Emission line spectra for selected elements) to observe the emission spectra for known. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From mungfali.com
Atomic Emission Spectrum Of Elements Spectroscopy A Virtual Lab Element Identification And Emission Spectra The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Use the virtual spectroscopy lab (part 2: Different elements produce different spectra that are unique enough to be. These radiations are seen in the form of. Emission line spectra for selected elements) to observe. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From cz.pinterest.com
You Can Download Inspirational Periodic Table Virtual Lab At here https Spectroscopy A Virtual Lab Element Identification And Emission Spectra Use the virtual spectroscopy lab (part 2: Emission line spectra for selected elements) to observe the emission spectra for known. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. When hydrogen gas is placed. Different elements produce different spectra that are unique enough to be. 1.observe the bright line spectra (emission spectra) for various elements. When. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.resonancescience.org
What is Resonance and Why is it so Important? Spectroscopy A Virtual Lab Element Identification And Emission Spectra The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. Spectroscope you can view the emission line spectra. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. 1.observe the bright line spectra. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.slideserve.com
PPT Introduction to Spectroscopy PowerPoint Presentation, free Spectroscopy A Virtual Lab Element Identification And Emission Spectra The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When an atom or ion in substance got excited, they emit radiations of particular frequencies. When hydrogen gas is placed. Emission line spectra for selected elements) to observe the emission spectra for known. Different. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.youtube.com
5.3 Atomic Emission Spectra & the Quantum Mechanical Model YouTube Spectroscopy A Virtual Lab Element Identification And Emission Spectra 1.observe the bright line spectra (emission spectra) for various elements. Emission line spectra for selected elements) to observe the emission spectra for known. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. When an atom or ion in substance got excited, they emit radiations of particular frequencies. The emission spectrum (or line spectrum) of a chemical. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Spectroscopy A Virtual Lab Element Identification And Emission Spectra When an atom or ion in substance got excited, they emit radiations of particular frequencies. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light.. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.mrpalermo.com
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom Spectroscopy A Virtual Lab Element Identification And Emission Spectra When hydrogen gas is placed. 1.observe the bright line spectra (emission spectra) for various elements. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Emission line spectra for selected elements) to observe the emission spectra for known. Use the virtual spectroscopy lab (part 2: A virtual lab element identification and emission spectra what you need to. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From studylib.net
Copy of Virtual Lab Atomic Emission Spectra Spectroscopy A Virtual Lab Element Identification And Emission Spectra 2.use a flame test to observe the color produced when metal ions. Spectroscope you can view the emission line spectra. Use the virtual spectroscopy lab (part 2: A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the key to. 1.observe the bright line spectra (emission spectra) for various. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.sliderbase.com
Emission spectra Presentation Physics Spectroscopy A Virtual Lab Element Identification And Emission Spectra The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. These radiations are seen in the form of. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. Spectroscope you can view the emission line spectra. 2.use a flame test to observe the color produced when. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From umop.net
Visible Spectra of the Elements Spectroscopy A Virtual Lab Element Identification And Emission Spectra When an atom or ion in substance got excited, they emit radiations of particular frequencies. Spectroscope you can view the emission line spectra. 2.use a flame test to observe the color produced when metal ions. When hydrogen gas is placed. Emission line spectra for selected elements) to observe the emission spectra for known. A virtual lab element identification and emission. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.coe.edu
Spectroscopy Coe College Spectroscopy A Virtual Lab Element Identification And Emission Spectra Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 1.observe the bright line spectra (emission spectra) for various elements. The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. Use the virtual spectroscopy lab (part 2: These radiations are seen in the form of. 2.use. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From universetoday.com
Spectroscopy The Key to Humanity’s Future in Space Spectroscopy A Virtual Lab Element Identification And Emission Spectra Spectroscope you can view the emission line spectra. Emission line spectra for selected elements) to observe the emission spectra for known. When an atom or ion in substance got excited, they emit radiations of particular frequencies. The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. A virtual lab. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Spectroscopy A Virtual Lab Element Identification And Emission Spectra Spectroscope you can view the emission line spectra. 2.use a flame test to observe the color produced when metal ions. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The unique color that is observed during a flame test is actually a mixture. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From uwaterloo.ca
Periodic Table of emission spectra Chem 13 News Magazine University Spectroscopy A Virtual Lab Element Identification And Emission Spectra When hydrogen gas is placed. 2.use a flame test to observe the color produced when metal ions. Emission line spectra for selected elements) to observe the emission spectra for known. Different elements produce different spectra that are unique enough to be. 1.observe the bright line spectra (emission spectra) for various elements. A virtual lab element identification and emission spectra what. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.mrpalermo.com
Virtual Lab Flame Test & Spectroscopy Mr. Palermo's Flipped Spectroscopy A Virtual Lab Element Identification And Emission Spectra When hydrogen gas is placed. The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 1.observe the bright line spectra (emission spectra) for various elements. Emission line spectra for selected elements) to observe the emission spectra. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.thoughtco.com
What Is Luminosity and What does it Tell Us? Spectroscopy A Virtual Lab Element Identification And Emission Spectra Use the virtual spectroscopy lab (part 2: The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Different elements produce different spectra that. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.chegg.com
Solved Spectroscopy. A Virtual Lab Element Identification Spectroscopy A Virtual Lab Element Identification And Emission Spectra The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Use the virtual spectroscopy lab (part 2: These radiations are seen in the form of. 1.observe the bright line spectra (emission spectra) for various elements. When an atom or ion in substance got excited,. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From studylib.net
Spectroscopy Element Identification and Emission Spectra Spectroscopy A Virtual Lab Element Identification And Emission Spectra The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When an atom or ion in substance got excited, they emit radiations of particular frequencies. A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.
From www.slideserve.com
PPT Atomic Emission Spectra PowerPoint Presentation, free download Spectroscopy A Virtual Lab Element Identification And Emission Spectra The unique color that is observed during a flame test is actually a mixture of several different wavelengths of visible light. 1.observe the bright line spectra (emission spectra) for various elements. Use the virtual spectroscopy lab (part 2: A virtual lab element identification and emission spectra what you need to know the energy levels in atoms and ions are the. Spectroscopy A Virtual Lab Element Identification And Emission Spectra.