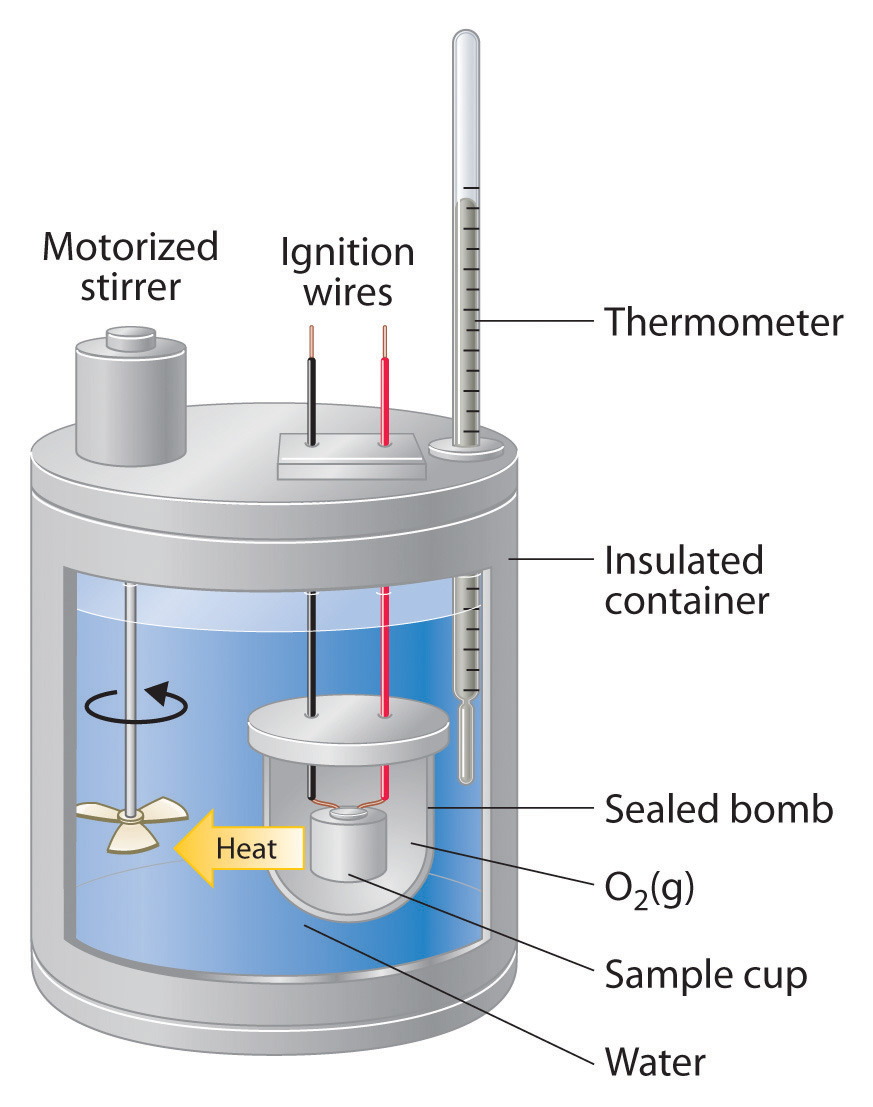
How Does A Calorimeter Measure The Heat Content In Food . we measure the energy in food through a process known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. The temperature change of the water,. To do so, the heat is exchanged with a calibrated object. This method involves measuring the heat released by. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. calorimetry is used to measure amounts of heat transferred to or from a substance.
from chemwiki.ucdavis.edu
To do so, the heat is exchanged with a. To do so, the heat is exchanged with a calibrated object. To do so, the heat is exchanged with a calibrated object. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. we measure the energy in food through a process known as calorimetry. The temperature change of the water,. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
Chapter 9.6 Calorimetry Chemwiki
How Does A Calorimeter Measure The Heat Content In Food calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. The temperature change of the water,. calorimetry is used to measure amounts of heat transferred to or from a substance. This method involves measuring the heat released by. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. we measure the energy in food through a process known as calorimetry. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. To do so, the heat is exchanged with a. To do so, the heat is exchanged with a calibrated object. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.slideserve.com
PPT Chapter 2 Energy and Matter PowerPoint Presentation, free download ID4667074 How Does A Calorimeter Measure The Heat Content In Food a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. we measure the energy in food through a process. How Does A Calorimeter Measure The Heat Content In Food.
From www.youtube.com
Thermodynamics Calculate Energy Content of Food from Calorimeter YouTube How Does A Calorimeter Measure The Heat Content In Food a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is. How Does A Calorimeter Measure The Heat Content In Food.
From dxobmbkmm.blob.core.windows.net
Bomb Calorimeter Used To Measure at Angela Johnson blog How Does A Calorimeter Measure The Heat Content In Food To do so, the heat is exchanged with a. To do so, the heat is exchanged with a calibrated object. The temperature change of the water,. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. To do so, the heat is exchanged with a calibrated object. calorimetry is used. How Does A Calorimeter Measure The Heat Content In Food.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 How Does A Calorimeter Measure The Heat Content In Food we measure the energy in food through a process known as calorimetry. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. To do. How Does A Calorimeter Measure The Heat Content In Food.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? How Does A Calorimeter Measure The Heat Content In Food a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. This method involves measuring the heat released by. a calorimeter is a device. How Does A Calorimeter Measure The Heat Content In Food.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica How Does A Calorimeter Measure The Heat Content In Food we measure the energy in food through a process known as calorimetry. This method involves measuring the heat released by. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. To do so, the heat is exchanged with a calibrated object. calorimetry. How Does A Calorimeter Measure The Heat Content In Food.
From www.animalia-life.club
Food Calorimeter How Does A Calorimeter Measure The Heat Content In Food This method involves measuring the heat released by. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The temperature change of the water,. To do so, the heat is exchanged with a. calorimetry. How Does A Calorimeter Measure The Heat Content In Food.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download ID4784803 How Does A Calorimeter Measure The Heat Content In Food To do so, the heat is exchanged with a. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. we measure the energy in food through a process. How Does A Calorimeter Measure The Heat Content In Food.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience How Does A Calorimeter Measure The Heat Content In Food a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The temperature change of the water,. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used. How Does A Calorimeter Measure The Heat Content In Food.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki How Does A Calorimeter Measure The Heat Content In Food calorimetry is used to measure amounts of heat transferred to or from a substance. we measure the energy in food through a process known as calorimetry. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical. How Does A Calorimeter Measure The Heat Content In Food.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts How Does A Calorimeter Measure The Heat Content In Food To do so, the heat is exchanged with a. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. calorimetry is used to measure amounts of heat transferred to or. How Does A Calorimeter Measure The Heat Content In Food.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses How Does A Calorimeter Measure The Heat Content In Food calorimetry is used to measure amounts of heat transferred to or from a substance. we measure the energy in food through a process known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or. How Does A Calorimeter Measure The Heat Content In Food.
From www.thoughtco.com
Calorimeter Definition in Chemistry How Does A Calorimeter Measure The Heat Content In Food a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. we measure the energy in food through a process known as calorimetry. To do so, the heat is exchanged with a calibrated object. To. How Does A Calorimeter Measure The Heat Content In Food.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab How Does A Calorimeter Measure The Heat Content In Food calorimetry is used to measure amounts of heat transferred to or from a substance. The temperature change of the water,. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to. How Does A Calorimeter Measure The Heat Content In Food.
From www.numerade.com
SOLVED A bomb calorimeter or a constant volume calorimeler; is a device often used to How Does A Calorimeter Measure The Heat Content In Food This method involves measuring the heat released by. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a. calorimetry is used. How Does A Calorimeter Measure The Heat Content In Food.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID6317093 How Does A Calorimeter Measure The Heat Content In Food This method involves measuring the heat released by. To do so, the heat is exchanged with a. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is. How Does A Calorimeter Measure The Heat Content In Food.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 How Does A Calorimeter Measure The Heat Content In Food we measure the energy in food through a process known as calorimetry. This method involves measuring the heat released by. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the. How Does A Calorimeter Measure The Heat Content In Food.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 How Does A Calorimeter Measure The Heat Content In Food To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. This method involves measuring the heat released by. calorimetry is used to measure amounts of heat transferred to or from a substance. The temperature change of the water,. To do so, the heat. How Does A Calorimeter Measure The Heat Content In Food.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of How Does A Calorimeter Measure The Heat Content In Food a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. The temperature change of the water,. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is. How Does A Calorimeter Measure The Heat Content In Food.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry How Does A Calorimeter Measure The Heat Content In Food a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. The temperature change of the water,. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. we measure the energy in food through a process known. How Does A Calorimeter Measure The Heat Content In Food.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog How Does A Calorimeter Measure The Heat Content In Food To do so, the heat is exchanged with a calibrated object. The temperature change of the water,. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is usually an isolated environment. How Does A Calorimeter Measure The Heat Content In Food.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types, Examples and Uses CollegeSearch How Does A Calorimeter Measure The Heat Content In Food To do so, the heat is exchanged with a. The temperature change of the water,. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is usually an isolated environment in which. How Does A Calorimeter Measure The Heat Content In Food.
From www.animalia-life.club
Calorimeter Diagram How Does A Calorimeter Measure The Heat Content In Food To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. This method involves measuring the heat released by. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be. How Does A Calorimeter Measure The Heat Content In Food.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry How Does A Calorimeter Measure The Heat Content In Food we measure the energy in food through a process known as calorimetry. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. This method involves measuring the heat released by. To do so, the heat is exchanged with a. The temperature change of the water,. To do so, the heat. How Does A Calorimeter Measure The Heat Content In Food.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube How Does A Calorimeter Measure The Heat Content In Food calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. we measure the energy in food through a process known as calorimetry. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to. How Does A Calorimeter Measure The Heat Content In Food.
From www.carolina.com
Food Calorimetry How to Measure Calories in Food How Does A Calorimeter Measure The Heat Content In Food To do so, the heat is exchanged with a. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. . How Does A Calorimeter Measure The Heat Content In Food.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Does A Calorimeter Measure The Heat Content In Food This method involves measuring the heat released by. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. we measure the energy in food through a process known as calorimetry. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of. How Does A Calorimeter Measure The Heat Content In Food.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses How Does A Calorimeter Measure The Heat Content In Food calorimetry is used to measure amounts of heat transferred to or from a substance. we measure the energy in food through a process known as calorimetry. To do so, the heat is exchanged with a. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. To do so, the. How Does A Calorimeter Measure The Heat Content In Food.
From www.slideserve.com
PPT A calorimeter is used to measure the amount of heat absorbed or released during a chemical How Does A Calorimeter Measure The Heat Content In Food calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured.. How Does A Calorimeter Measure The Heat Content In Food.
From www.thermal-engineering.org
How does a calorimeter measure heat How Does A Calorimeter Measure The Heat Content In Food a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. To do so, the heat is exchanged with a. we measure the energy in food through a process known as calorimetry. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used. How Does A Calorimeter Measure The Heat Content In Food.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 How Does A Calorimeter Measure The Heat Content In Food a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. To do so, the heat is exchanged with a calibrated. How Does A Calorimeter Measure The Heat Content In Food.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation & Application How Does A Calorimeter Measure The Heat Content In Food This method involves measuring the heat released by. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so,. How Does A Calorimeter Measure The Heat Content In Food.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience How Does A Calorimeter Measure The Heat Content In Food calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. calorimetry is used to measure. How Does A Calorimeter Measure The Heat Content In Food.
From www.slideshare.net
Lesson Enthalpy and Calorimetry How Does A Calorimeter Measure The Heat Content In Food calorimetry is used to measure amounts of heat transferred to or from a substance. The temperature change of the water,. To do so, the heat is exchanged with a calibrated object. This method involves measuring the heat released by. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat. How Does A Calorimeter Measure The Heat Content In Food.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry How Does A Calorimeter Measure The Heat Content In Food a calorimeter is usually an isolated environment in which the heat absorbed or generated can be accurately measured. The temperature change of the water,. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. This method involves measuring the heat released by. . How Does A Calorimeter Measure The Heat Content In Food.