What Is The Ph Of Sodium Fluoride . explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. Visit byju's to understand the properties, structure and its uses. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known.
from www.chegg.com
Visit byju's to understand the properties, structure and its uses. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7.
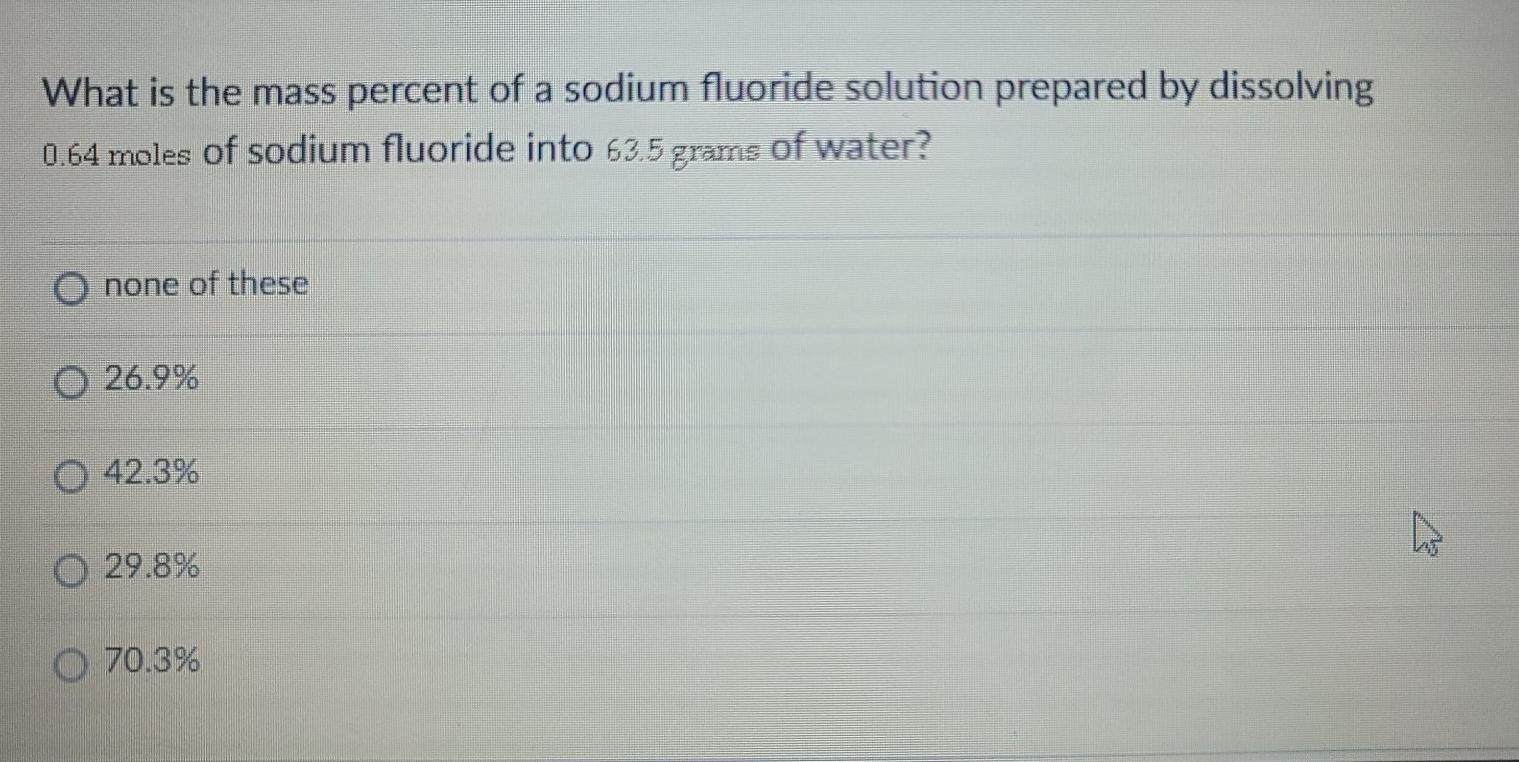
Solved What is the mass percent of a sodium fluoride
What Is The Ph Of Sodium Fluoride — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. Visit byju's to understand the properties, structure and its uses. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known.
From www.researchgate.net
A schematic showing the process of producing sodium fluoride solutions. Download Scientific What Is The Ph Of Sodium Fluoride a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. — the ph of the resulting solution can be determined if the kb. What Is The Ph Of Sodium Fluoride.
From openi.nlm.nih.gov
19F NMR spectra of sodium fluoride titrated against Ccl Openi What Is The Ph Of Sodium Fluoride a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth,. What Is The Ph Of Sodium Fluoride.
From pixels.com
Sodium Fluoride Salt Chemical Structure Photograph by Molekuul/science Photo Library Pixels What Is The Ph Of Sodium Fluoride — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. Visit byju's to understand the properties, structure and its uses. explore sodium fluoride’s. What Is The Ph Of Sodium Fluoride.
From www.chegg.com
Solved What is the mass percent of a sodium fluoride What Is The Ph Of Sodium Fluoride sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. Visit byju's to understand the properties, structure and its uses. — the ph of a sodium fluoride solution can be. What Is The Ph Of Sodium Fluoride.
From www.alamy.com
Sodium fluoride salt, chemical structure. White skeletal formula on dark teal gradient What Is The Ph Of Sodium Fluoride explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. Visit byju's to understand the properties, structure and its uses. a salt that is derived from the reaction of a strong acid with a. What Is The Ph Of Sodium Fluoride.
From fineartamerica.com
Sodium Fluoride Salt Chemical Structure Photograph by Molekuul/science Photo Library Fine Art What Is The Ph Of Sodium Fluoride — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. a salt that is. What Is The Ph Of Sodium Fluoride.
From alkalinewaterplus.blogspot.com
Alkaline Ionized Water Learning About Water Ionizers March 2015 What Is The Ph Of Sodium Fluoride explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. Visit byju's to understand the properties, structure and its uses. — the ph of a sodium fluoride solution can be calculated using the dissociation. What Is The Ph Of Sodium Fluoride.
From www.dreamstime.com
Sodium Fluoride Ionic Compound Created Stock Vector Illustration of mobility, crystal 195444456 What Is The Ph Of Sodium Fluoride — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. Visit byju's to understand the properties, structure and its uses. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and. What Is The Ph Of Sodium Fluoride.
From www.alamy.com
Sodium fluoride, chemical structure. 3D rendering. Atoms are represented as spheres with What Is The Ph Of Sodium Fluoride — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. Visit byju's to understand the properties, structure and its uses. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks,. What Is The Ph Of Sodium Fluoride.
From www.shutterstock.com
Sodium Fluoride Chemical Structure Skeletal Formula Stock Illustration 1306674934 Shutterstock What Is The Ph Of Sodium Fluoride Visit byju's to understand the properties, structure and its uses. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of the resulting solution can be determined if the kb of the. What Is The Ph Of Sodium Fluoride.
From www.dreamstime.com
Sodium Fluoride, Chemical Structure. 3D Rendering Stock Illustration Illustration of model What Is The Ph Of Sodium Fluoride Visit byju's to understand the properties, structure and its uses. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — the ph of. What Is The Ph Of Sodium Fluoride.
From www.sciencephoto.com
Sodium fluoride salt chemical structure, illustration Stock Image F027/9448 Science Photo What Is The Ph Of Sodium Fluoride explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. — the ph of a. What Is The Ph Of Sodium Fluoride.
From sweetfriends.co.nz
Fluoride Conversion Chart Xylitol Sugar Free Sweet Friends 0800 289 777 What Is The Ph Of Sodium Fluoride explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. a salt that is derived. What Is The Ph Of Sodium Fluoride.
From www.numerade.com
Calculate the pH of a buffer solution made from 0.30 M hydrofluoric acid and 0.70 M sodium What Is The Ph Of Sodium Fluoride sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — the ph of the resulting solution can be determined if the kb of. What Is The Ph Of Sodium Fluoride.
From pixels.com
Sodium Fluoride Photograph by Molekuul/science Photo Library Pixels What Is The Ph Of Sodium Fluoride explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization.. What Is The Ph Of Sodium Fluoride.
From molekula.com
Purchase Sodium fluoride [7681494] online • Catalog • Molekula Group What Is The Ph Of Sodium Fluoride Visit byju's to understand the properties, structure and its uses. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. a salt that is derived from the reaction of. What Is The Ph Of Sodium Fluoride.
From www.carolina.com
Sodium Fluoride, Powder, Laboratory Grade, 100 g Carolina Biological Supply What Is The Ph Of Sodium Fluoride a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization.. What Is The Ph Of Sodium Fluoride.
From www.researchgate.net
A schematic showing the process of producing sodium fluoride solutions. Download Scientific What Is The Ph Of Sodium Fluoride a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride. What Is The Ph Of Sodium Fluoride.
From www.semanticscholar.org
Table 1 from Effects of pH on Expression of Sodium Fluoride Resistance in Streptococcus mutans What Is The Ph Of Sodium Fluoride — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. Visit byju's to understand the properties, structure and its uses. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks,. What Is The Ph Of Sodium Fluoride.
From ar.inspiredpencil.com
Sodium Fluoride Molecule What Is The Ph Of Sodium Fluoride — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. Visit byju's to understand the properties, structure and its uses. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and. What Is The Ph Of Sodium Fluoride.
From www.differencebetween.com
Difference Between Sodium Fluoride and Sodium Monofluorophosphate Compare the Difference What Is The Ph Of Sodium Fluoride a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. — the ph of a sodium fluoride solution can be calculated using the. What Is The Ph Of Sodium Fluoride.
From www.shutterstock.com
Sodium Fluoride Properties Chemical Compound Structure Stock Vector (Royalty Free) 1978737572 What Is The Ph Of Sodium Fluoride — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. — the ph of a. What Is The Ph Of Sodium Fluoride.
From en.wikipedia.org
Sodium fluoride Wikipedia What Is The Ph Of Sodium Fluoride sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. — the ph of the. What Is The Ph Of Sodium Fluoride.
From www.researchgate.net
Influence of pH on the precipitation of fluorides and phosphates from... Download Scientific What Is The Ph Of Sodium Fluoride a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. — the ph of the resulting solution can be determined if the kb. What Is The Ph Of Sodium Fluoride.
From pediaa.com
Difference Between Sodium Fluoride and Fluoride Definition, Properties, Reactions What Is The Ph Of Sodium Fluoride a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. Visit byju's to understand the properties, structure and its uses. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. explore sodium fluoride’s. What Is The Ph Of Sodium Fluoride.
From www.worksheetsplanet.com
What is PH Definition What Is The Ph Of Sodium Fluoride — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. Visit byju's to understand the properties, structure and its uses. a salt that is derived from the reaction of. What Is The Ph Of Sodium Fluoride.
From www.numerade.com
A pure sample of Sodium fluoride (NaF) contains 69.0 grams of sodium. How many grams of fluorine What Is The Ph Of Sodium Fluoride a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this.. What Is The Ph Of Sodium Fluoride.
From www.numerade.com
SOLVED Calculate the pH of a buffer solution made from 0.30 M hydrofluoric acid and 0.70 M What Is The Ph Of Sodium Fluoride — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. Visit byju's to understand the properties, structure and its uses. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. sodium fluoride is. What Is The Ph Of Sodium Fluoride.
From pediaa.com
Difference Between Sodium Fluoride and Calcium Fluoride Definition, Chemical Properties What Is The Ph Of Sodium Fluoride explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. Visit byju's to understand the properties, structure and its uses. a salt that is derived from the reaction of a strong acid with a strong. What Is The Ph Of Sodium Fluoride.
From en.luminophor.ru
Sodium fluoride What Is The Ph Of Sodium Fluoride — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of. What Is The Ph Of Sodium Fluoride.
From stock.adobe.com
3D image of Sodium fluoride skeletal formula molecular chemical structure of What Is The Ph Of Sodium Fluoride Visit byju's to understand the properties, structure and its uses. — the ph of the resulting solution can be determined if the kb of the fluoride ion is known. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks,. What Is The Ph Of Sodium Fluoride.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 What Is The Ph Of Sodium Fluoride — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. Visit byju's to understand the properties, structure and its uses. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which. What Is The Ph Of Sodium Fluoride.
From www.youtube.com
How to write chemical formula Sodium FluorideChemical Formula Sodium FluorideSodium Fluoride What Is The Ph Of Sodium Fluoride — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. Visit byju's to understand the properties, structure and its uses. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of 7. — the ph. What Is The Ph Of Sodium Fluoride.
From stock.adobe.com
Sodium fluoride, chemical structure. Skeletal formula. Stock Vector Adobe Stock What Is The Ph Of Sodium Fluoride explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of a sodium fluoride solution can be calculated using the dissociation constant of the fluoride ion. a salt that is derived from the reaction of a strong acid with a strong base forms a solution that has a ph of. What Is The Ph Of Sodium Fluoride.
From www.loudwolf.com
Sodium Fluoride What Is The Ph Of Sodium Fluoride sodium fluoride is absorbed by the surface of hydroxyapatite crystals on the teeth, which are necessary for mineralization. Visit byju's to understand the properties, structure and its uses. explore sodium fluoride’s chemical properties, synthesis, uses, potential risks, and regulatory guidelines in this. — the ph of the resulting solution can be determined if the kb of the. What Is The Ph Of Sodium Fluoride.