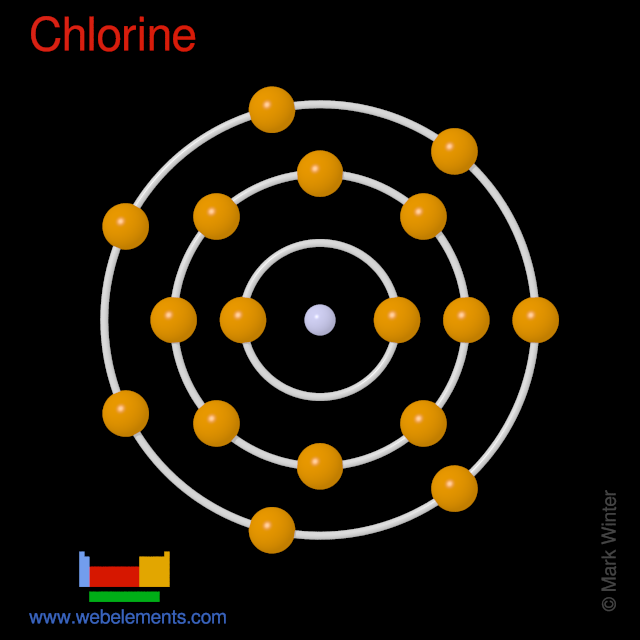
Atom Chlorine Ground State . Add an electron to the anion. if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. How many valence electrons are found in a neutral ground. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. let's find the ground state electron configuration of chlorine! The ground state is the most stable energy state for. Full ground state electron configuration: the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5.
from www.webelements.com
if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: The ground state is the most stable energy state for. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. let's find the ground state electron configuration of chlorine! How many valence electrons are found in a neutral ground. Full ground state electron configuration: Add an electron to the anion. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17.
Elements Periodic Table » Chlorine » properties of free atoms
Atom Chlorine Ground State How many valence electrons are found in a neutral ground. let's find the ground state electron configuration of chlorine! How many valence electrons are found in a neutral ground. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. The ground state is the most stable energy state for. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Full ground state electron configuration: if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. Add an electron to the anion.
From exyccngyt.blob.core.windows.net
Chlorine Structure Type at Robert Bates blog Atom Chlorine Ground State if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. How many valence electrons are found in a neutral ground. draw an orbital. Atom Chlorine Ground State.
From download4update.blogspot.com
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio Atom Chlorine Ground State Full ground state electron configuration: if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. The. Atom Chlorine Ground State.
From ecurrencythailand.com
When A Chlorine Atom Forms An Ion Its Radius? 10 Most Correct Answers Atom Chlorine Ground State let's find the ground state electron configuration of chlorine! How many valence electrons are found in a neutral ground. Add an electron to the anion. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Full ground state electron configuration: draw an orbital diagram and use it to derive the nobel gas electron. Atom Chlorine Ground State.
From www.pinterest.com.mx
Pin en Stacey Atom Chlorine Ground State draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Full ground state electron configuration: . Atom Chlorine Ground State.
From www.youtube.com
Complete the atomic orbital diagram for the groundstate electronic Atom Chlorine Ground State Full ground state electron configuration: How many valence electrons are found in a neutral ground. let's find the ground state electron configuration of chlorine! the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. Add an electron to the anion. if an atom has lost or gained electrons by changing to a cation or anion, follow. Atom Chlorine Ground State.
From www.youtube.com
Term symbol of Chlorine atom in ground state. Mock test No. 5 on Atom Chlorine Ground State How many valence electrons are found in a neutral ground. Full ground state electron configuration: let's find the ground state electron configuration of chlorine! draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures.. Atom Chlorine Ground State.
From www.animalia-life.club
Chlorine Bohr Model Atom Chlorine Ground State if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. let's find the ground state electron configuration of chlorine! the ground state configuration of the neutral chlorine atom is. Atom Chlorine Ground State.
From ar.inspiredpencil.com
Chlorine Atom Diagram Atom Chlorine Ground State let's find the ground state electron configuration of chlorine! if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Add an electron to the anion. How many valence electrons are found in a neutral ground. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. Full ground state. Atom Chlorine Ground State.
From www.numerade.com
SOLVED Complete the atomic orbital diagram for the groundstate Atom Chlorine Ground State draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. let's find the ground state electron configuration of chlorine! ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. if an atom has lost or gained electrons by changing to a cation or. Atom Chlorine Ground State.
From www.numerade.com
SOLVEDA chlorine atom in its ground state has a total of seven Atom Chlorine Ground State let's find the ground state electron configuration of chlorine! ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Add an electron to the anion. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. the ground state configuration of the neutral chlorine. Atom Chlorine Ground State.
From circuitwiringreseau123.z22.web.core.windows.net
Helium Electron Dot Diagram Atom Chlorine Ground State ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. The ground state is the most stable energy state for. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. Add an electron. Atom Chlorine Ground State.
From www.numerade.com
SOLVED Complete the atomic orbital diagram for the groundstate Atom Chlorine Ground State How many valence electrons are found in a neutral ground. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. if an atom has lost or gained electrons by changing to a cation or anion, follow these. Atom Chlorine Ground State.
From ar.inspiredpencil.com
Bohr Model Chlorine Atom Chlorine Ground State The ground state is the most stable energy state for. let's find the ground state electron configuration of chlorine! Full ground state electron configuration: if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. How. Atom Chlorine Ground State.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms Atom Chlorine Ground State let's find the ground state electron configuration of chlorine! How many valence electrons are found in a neutral ground. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. if an atom has lost or gained. Atom Chlorine Ground State.
From www.animalia-life.club
Chlorine Bohr Model Atom Chlorine Ground State ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The ground state is the most stable energy state for. if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Full ground state electron configuration: the ground state configuration of the neutral chlorine atom is. Atom Chlorine Ground State.
From www.dreamstime.com
Chlorine Atom, with Mass and Energy Levels. Stock Vector Illustration Atom Chlorine Ground State Full ground state electron configuration: draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. How many valence electrons are found in a neutral ground. let's find the ground state electron configuration of chlorine! if an atom has lost or gained electrons by changing to a cation or. Atom Chlorine Ground State.
From www.toppr.com
Chlorine atom in its third excited state with fluorine to form a Atom Chlorine Ground State if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Full ground state electron configuration: let's find the ground state electron configuration of chlorine! The ground state is the most stable energy state for. How many valence electrons are found in a neutral ground. ground state electron configurations are. Atom Chlorine Ground State.
From exywbfeiw.blob.core.windows.net
Chlorine Electron Configuration Class 9 at Carol Hollenbeck blog Atom Chlorine Ground State let's find the ground state electron configuration of chlorine! How many valence electrons are found in a neutral ground. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. Full ground state electron configuration: ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures.. Atom Chlorine Ground State.
From atomic-no-of-chlorine.peatix.com
Atomic No Of Chlorine Peatix Atom Chlorine Ground State How many valence electrons are found in a neutral ground. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. if an atom has lost or gained electrons by changing to a cation or anion, follow these. Atom Chlorine Ground State.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Atom Chlorine Ground State How many valence electrons are found in a neutral ground. The ground state is the most stable energy state for. Full ground state electron configuration: ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: . Atom Chlorine Ground State.
From www.animalia-life.club
Chlorine Bohr Model Atom Chlorine Ground State How many valence electrons are found in a neutral ground. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. The ground state is the most stable energy state for. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. let's find the ground state electron configuration. Atom Chlorine Ground State.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Atom Chlorine Ground State let's find the ground state electron configuration of chlorine! The ground state is the most stable energy state for. if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Add an electron to the anion. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures.. Atom Chlorine Ground State.
From gioqgskgi.blob.core.windows.net
Chlorine And Atomic Mass at Oscar Parks blog Atom Chlorine Ground State ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. How many valence electrons are found in a neutral ground. Add an electron to the anion. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. let's find the ground state electron configuration of chlorine! The ground state is the most stable. Atom Chlorine Ground State.
From valenceelectrons.com
How to Write the Electron Configuration for Chlorine (Cl)? Atom Chlorine Ground State How many valence electrons are found in a neutral ground. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: let's find the ground state electron configuration of chlorine! Full ground state electron configuration: . Atom Chlorine Ground State.
From fyoujeahv.blob.core.windows.net
Chlorine Atom Has Protons at Linda Kraft blog Atom Chlorine Ground State ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. Add an electron to the anion. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. let's find the ground state electron. Atom Chlorine Ground State.
From www.youtube.com
Chlorine Ground State Electron Configuration YouTube Atom Chlorine Ground State draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. The ground state is the most stable energy state for. Full ground state electron configuration: let's find the ground state electron configuration of chlorine! ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures.. Atom Chlorine Ground State.
From www.researchgate.net
(PDF) On the influence of rare gas atomchlorine ion potentials on the Atom Chlorine Ground State if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: Full ground state electron configuration: ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. let's find the ground state electron configuration of chlorine! Add an electron to the anion. The ground state is the. Atom Chlorine Ground State.
From loeaigewr.blob.core.windows.net
Chlorine Complete Ground State Electron Configuration at Garret Sanders Atom Chlorine Ground State Add an electron to the anion. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. let's find. Atom Chlorine Ground State.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Atom Chlorine Ground State let's find the ground state electron configuration of chlorine! if an atom has lost or gained electrons by changing to a cation or anion, follow these steps: ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. The ground state is. Atom Chlorine Ground State.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Atom Chlorine Ground State How many valence electrons are found in a neutral ground. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The ground state is the most stable energy state for. Add an electron to the anion. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z =. Atom Chlorine Ground State.
From www.schoolmykids.com
Compare Chlorine vs Indium Periodic Table Element Comparison Atom Chlorine Ground State ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. if an atom has lost or gained electrons by changing to a. Atom Chlorine Ground State.
From imgbin.com
Lewis Structure Electron Chlorine Diagram Chloride PNG, Clipart, Black Atom Chlorine Ground State draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. The ground state is the most stable energy state for. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. let's find the ground state electron configuration of chlorine! Add an electron to the. Atom Chlorine Ground State.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Atom Chlorine Ground State The ground state is the most stable energy state for. let's find the ground state electron configuration of chlorine! the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. How many valence electrons are found in a neutral ground. ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Full ground state. Atom Chlorine Ground State.
From fyombkdzq.blob.core.windows.net
Chlorine Ion Has at Kim Jones blog Atom Chlorine Ground State Add an electron to the anion. Full ground state electron configuration: ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. The ground state is the most stable energy state for. let's find the. Atom Chlorine Ground State.
From www.youtube.com
Chlorine Electron Configuration YouTube Atom Chlorine Ground State let's find the ground state electron configuration of chlorine! Add an electron to the anion. The ground state is the most stable energy state for. draw an orbital diagram and use it to derive the nobel gas electron configuration of chlorine, z = 17. the ground state configuration of the neutral chlorine atom is 1s22s22p63s23p5. ground. Atom Chlorine Ground State.