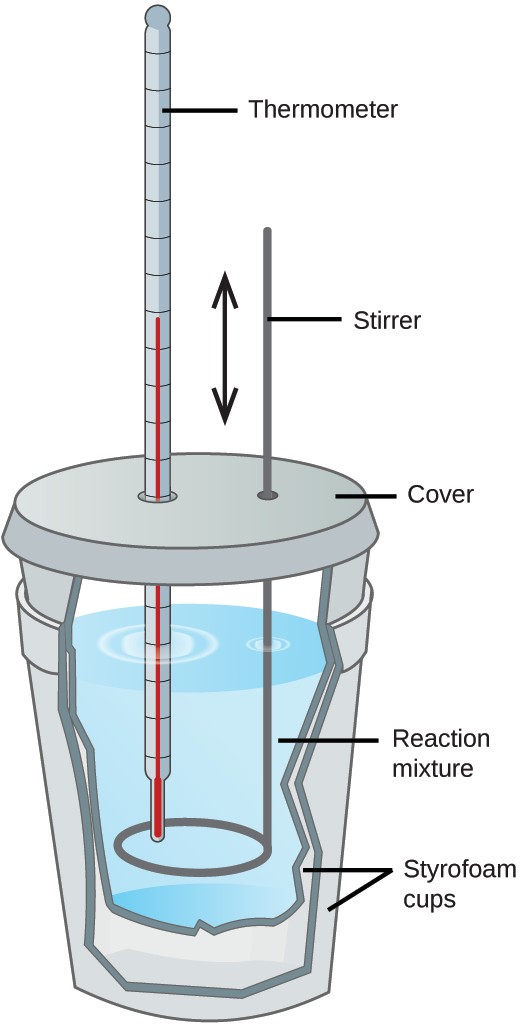
Calorimetry Lab Pdf . Measuring the latent heat of fusion of water, and measuring. As part of this lab, you will: Lab session 9, experiment 8: In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Find the heat capacity (cp) of a calorimeter and contents. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Measure the amount of heat involved in frostbite. In this lab, you will do two classic calorimetry experiments: The heat evolved by a chemical reaction can be determined using a calorimeter. The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. This experiment has three primary objectives: Chemical reactions involve the release or consumption of energy, usually in the.
from louis.pressbooks.pub
Measuring the latent heat of fusion of water, and measuring. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. In this lab, you will do two classic calorimetry experiments: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Chemical reactions involve the release or consumption of energy, usually in the. This experiment has three primary objectives: The heat evolved by a chemical reaction can be determined using a calorimeter. Measure the amount of heat involved in frostbite. Lab session 9, experiment 8: As part of this lab, you will:
Calorimetry (9.2) General Chemistry
Calorimetry Lab Pdf Find the heat capacity (cp) of a calorimeter and contents. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. This experiment has three primary objectives: In this lab, you will do two classic calorimetry experiments: Lab session 9, experiment 8: Chemical reactions involve the release or consumption of energy, usually in the. Measure the amount of heat involved in frostbite. As part of this lab, you will: The heat evolved by a chemical reaction can be determined using a calorimeter. Find the heat capacity (cp) of a calorimeter and contents. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. Measuring the latent heat of fusion of water, and measuring.
From pdfprof.com
experiment 25 calorimetry lab report Calorimetry Lab Pdf Measuring the latent heat of fusion of water, and measuring. Chemical reactions involve the release or consumption of energy, usually in the. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid. Calorimetry Lab Pdf.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog Calorimetry Lab Pdf Lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. This experiment has three primary objectives: In this lab, you will do two classic calorimetry experiments: Measuring the latent heat of fusion of water, and measuring. In calorimetry, an insulated reaction container, called a calorimeter,. Calorimetry Lab Pdf.
From www.scribd.com
Calorimetry Lab PDF Calorimetry Lab Pdf The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. Measure the amount of heat involved in frostbite. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The heat evolved by a chemical reaction can be determined using. Calorimetry Lab Pdf.
From users.highland.edu
Calorimetry Calorimetry Lab Pdf Measuring the latent heat of fusion of water, and measuring. The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Lab session 9, experiment 8: Chemical reactions involve the. Calorimetry Lab Pdf.
From www.pdfprof.com
calorimétrie pdf Calorimetry Lab Pdf In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. As part of this lab, you will: Find the heat capacity (cp) of a calorimeter and contents. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature.. Calorimetry Lab Pdf.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry Lab Pdf The heat evolved by a chemical reaction can be determined using a calorimeter. Find the heat capacity (cp) of a calorimeter and contents. Measure the amount of heat involved in frostbite. Chemical reactions involve the release or consumption of energy, usually in the. The transfer of heat or flow of heat is expressed as the change in enthalpy of a. Calorimetry Lab Pdf.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Calorimetry Lab Pdf Measure the amount of heat involved in frostbite. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The heat evolved by a chemical reaction can be determined using a calorimeter. In this lab, you will do two classic calorimetry experiments: The transfer of heat or flow of heat is. Calorimetry Lab Pdf.
From saylordotorg.github.io
Calorimetry Calorimetry Lab Pdf In this lab, you will do two classic calorimetry experiments: Find the heat capacity (cp) of a calorimeter and contents. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Measure the amount of heat involved in frostbite. Measuring the latent heat of fusion of water, and measuring. Chemical reactions. Calorimetry Lab Pdf.
From dokumen.tips
(PDF) Bomb Calorimeter Lab Sheet DOKUMEN.TIPS Calorimetry Lab Pdf The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. In this lab, you will do two classic calorimetry experiments: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Find the heat capacity (cp) of a calorimeter and. Calorimetry Lab Pdf.
From pdfprof.com
Experiment 6 ? Calorimetry Calorimetry Lab Pdf Measure the amount of heat involved in frostbite. As part of this lab, you will: Lab session 9, experiment 8: The heat evolved by a chemical reaction can be determined using a calorimeter. The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. Measuring the latent heat of fusion. Calorimetry Lab Pdf.
From www.scribd.com
Calorimetry Lab Heat Capacity Heat Calorimetry Lab Pdf As part of this lab, you will: In this lab, you will do two classic calorimetry experiments: Lab session 9, experiment 8: The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. This experiment has three primary objectives: Chemical reactions involve the release or consumption of energy, usually in. Calorimetry Lab Pdf.
From www.chegg.com
Solved Chapter 6 Calorimetry Worksheet Key Open the Calorimetry Lab Pdf The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. This experiment has three primary objectives: In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. In this lab, you will do two classic calorimetry experiments:. Calorimetry Lab Pdf.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Pdf Measuring the latent heat of fusion of water, and measuring. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Lab session 9, experiment 8: Chemical reactions involve the release or consumption of energy, usually in the. Find the heat capacity (cp) of a calorimeter and contents. The transfer of. Calorimetry Lab Pdf.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Calorimetry Lab Pdf As part of this lab, you will: Measuring the latent heat of fusion of water, and measuring. Measure the amount of heat involved in frostbite. Chemical reactions involve the release or consumption of energy, usually in the. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. This experiment has. Calorimetry Lab Pdf.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Lab Pdf The heat evolved by a chemical reaction can be determined using a calorimeter. Find the heat capacity (cp) of a calorimeter and contents. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Measuring the latent heat of fusion of water, and measuring. The transfer of heat. Calorimetry Lab Pdf.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Lab Pdf In this lab, you will do two classic calorimetry experiments: Chemical reactions involve the release or consumption of energy, usually in the. The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. Find the heat capacity (cp) of a calorimeter and contents. Measure the amount of heat involved in. Calorimetry Lab Pdf.
From www.studypool.com
SOLUTION Student exploration calorimetry lab Studypool Calorimetry Lab Pdf Measure the amount of heat involved in frostbite. As part of this lab, you will: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. The heat. Calorimetry Lab Pdf.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Curtin Calorimetry Lab Pdf Lab session 9, experiment 8: In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. This experiment has three primary objectives: The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. Measuring the latent heat of. Calorimetry Lab Pdf.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Lab Pdf Measuring the latent heat of fusion of water, and measuring. In this lab, you will do two classic calorimetry experiments: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at. Calorimetry Lab Pdf.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Lab Pdf In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. As part of this lab, you will: In this lab, you will do two classic calorimetry experiments:. Calorimetry Lab Pdf.
From www.scribd.com
Lab 07Specific Heat & Calorimetry.pdf Heat Temperature Calorimetry Lab Pdf As part of this lab, you will: The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. The heat evolved by a chemical reaction can be determined using a calorimeter. Lab session 9, experiment 8: Find the heat capacity (cp) of a calorimeter and contents. In calorimetry, an insulated. Calorimetry Lab Pdf.
From www.scribd.com
Calorimetry Lab PDF Heat Quantity Calorimetry Lab Pdf In this lab, you will do two classic calorimetry experiments: In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. The heat evolved by a chemical reaction can be determined using a calorimeter. As part of this lab, you will: Measure the amount of heat involved in. Calorimetry Lab Pdf.
From www.pdfprof.com
experiment 25 calorimetry lab report Calorimetry Lab Pdf Chemical reactions involve the release or consumption of energy, usually in the. Measure the amount of heat involved in frostbite. The heat evolved by a chemical reaction can be determined using a calorimeter. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. As part of this. Calorimetry Lab Pdf.
From www.scribd.com
Lab 4 Calorimetry Lab PDF Temperature Heat Capacity Calorimetry Lab Pdf Find the heat capacity (cp) of a calorimeter and contents. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. The heat evolved by a chemical reaction can be determined using a calorimeter. As part of this lab, you will: Chemical reactions involve the release or consumption. Calorimetry Lab Pdf.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Lab Pdf Lab session 9, experiment 8: The heat evolved by a chemical reaction can be determined using a calorimeter. In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. As part of this lab, you will: In this lab, you will do two classic calorimetry experiments: Measure the. Calorimetry Lab Pdf.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Calorimetry Lab Pdf Measuring the latent heat of fusion of water, and measuring. Find the heat capacity (cp) of a calorimeter and contents. In this lab, you will do two classic calorimetry experiments: Measure the amount of heat involved in frostbite. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The transfer. Calorimetry Lab Pdf.
From www.scribd.com
Hand Out 1.5 Specific Heat Calorimetry Lab PDF Heat Calorimetry Calorimetry Lab Pdf In this lab, you will do two classic calorimetry experiments: In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. As part of this lab, you will: Lab session 9, experiment 8: Chemical reactions involve the release or consumption of energy, usually in the. This experiment has. Calorimetry Lab Pdf.
From lessonmagicrecharts.z13.web.core.windows.net
Calorimetry Questions And Answers Pdf Calorimetry Lab Pdf Measure the amount of heat involved in frostbite. This experiment has three primary objectives: Lab session 9, experiment 8: The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. In this lab, you will do two classic calorimetry experiments: Specific heat is an intensive property of a single phase. Calorimetry Lab Pdf.
From www.w3schools.blog
Cp, Cv calorimetry W3schools Calorimetry Lab Pdf Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. As part of this lab, you will: In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. Lab session 9, experiment 8: In this lab, you will. Calorimetry Lab Pdf.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry Calorimetry Lab Pdf Lab session 9, experiment 8: In this lab, you will do two classic calorimetry experiments: Chemical reactions involve the release or consumption of energy, usually in the. Find the heat capacity (cp) of a calorimeter and contents. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Measuring the latent. Calorimetry Lab Pdf.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student Calorimetry Lab Pdf The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. In this lab, you will do two classic calorimetry experiments: Lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. This experiment has three. Calorimetry Lab Pdf.
From www.pdfprof.com
determination of calorimeter constant lab report Calorimetry Lab Pdf Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. As part of this lab, you will: In calorimetry, an insulated reaction container, called a calorimeter, is filled with a measured amount of a liquid with a known heat capacity. The heat evolved by a chemical reaction can be determined. Calorimetry Lab Pdf.
From pdfprof.com
PDF Télécharger experiment 25 calorimetry pre lab answers Gratuit PDF Calorimetry Lab Pdf Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. This experiment has three primary objectives: Measuring the latent heat of fusion of water, and measuring. Measure the amount of heat involved in frostbite. As part of this lab, you will: In this lab, you will do two classic calorimetry. Calorimetry Lab Pdf.
From www.scribd.com
Calorimetry Lab Data Sheet PDF Branches Of Thermodynamics Heat Calorimetry Lab Pdf Lab session 9, experiment 8: The transfer of heat or flow of heat is expressed as the change in enthalpy of a reaction, ∆h, at constant. Chemical reactions involve the release or consumption of energy, usually in the. In this lab, you will do two classic calorimetry experiments: The heat evolved by a chemical reaction can be determined using a. Calorimetry Lab Pdf.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Pdf Measuring the latent heat of fusion of water, and measuring. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Find the heat capacity (cp) of a calorimeter and contents. Lab session 9, experiment 8: As part of this lab, you will: In calorimetry, an insulated reaction container, called a. Calorimetry Lab Pdf.