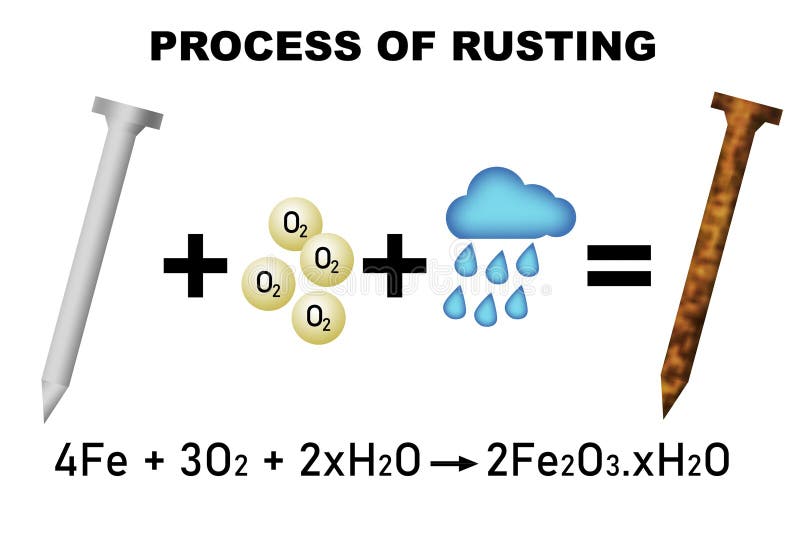
Copper Rust Formula . Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Rust forms when metal alloys containing iron undergo the. What is the chemical formula for corrosion in copper and silver?. Copper does not rust in water, but it can corrode. List some of the methods used to prevent or slow corrosion. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. Hint :metals when exposed to air or some chemicals for a long. Corrosion is usually defined as the degradation of metals due to an. When exposed to water, especially if it contains oxygen and other corrosive. After a few years, this tarnish gradually changes to dark brown or. To understand the process of corrosion. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. Rust is apparently a hydrated form of iron (iii)oxide.
from animalia-life.club
To understand the process of corrosion. Rust is apparently a hydrated form of iron (iii)oxide. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. Rust forms when metal alloys containing iron undergo the. List some of the methods used to prevent or slow corrosion. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Hint :metals when exposed to air or some chemicals for a long. After a few years, this tarnish gradually changes to dark brown or. When exposed to water, especially if it contains oxygen and other corrosive. Copper does not rust in water, but it can corrode.
Rusting Of Iron Chemical Reaction
Copper Rust Formula Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. What is the chemical formula for corrosion in copper and silver?. Rust is apparently a hydrated form of iron (iii)oxide. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. Corrosion is usually defined as the degradation of metals due to an. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. To understand the process of corrosion. Rust forms when metal alloys containing iron undergo the. Copper does not rust in water, but it can corrode. Hint :metals when exposed to air or some chemicals for a long. When exposed to water, especially if it contains oxygen and other corrosive. List some of the methods used to prevent or slow corrosion. After a few years, this tarnish gradually changes to dark brown or.
From pakistan.desertcart.com
Buy Flitz Brass and Copper Tarnish Remover, Powerful Formula That Copper Rust Formula When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. Rust forms when metal alloys containing iron undergo the. When exposed to water, especially if it contains oxygen and other corrosive. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. After a few years, this tarnish gradually changes to dark brown or.. Copper Rust Formula.
From www.youtube.com
How to Write the Formula for Copper (I) oxide YouTube Copper Rust Formula Copper does not rust in water, but it can corrode. List some of the methods used to prevent or slow corrosion. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Rust is apparently a hydrated form of iron (iii)oxide. To understand the process of corrosion. Rust forms when metal alloys containing iron undergo the. When exposed. Copper Rust Formula.
From wademcycarpenter.blogspot.com
Rusting Equation WademcyCarpenter Copper Rust Formula When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. Rust is apparently a hydrated form of iron (iii)oxide. Corrosion is usually defined as the degradation of metals due to an. To understand the process of corrosion. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i). Copper Rust Formula.
From www.tessshebaylo.com
Equation For Rusting Of Iron Tessshebaylo Copper Rust Formula What is the chemical formula for corrosion in copper and silver?. Rust forms when metal alloys containing iron undergo the. Corrosion is usually defined as the degradation of metals due to an. To understand the process of corrosion. Rust is apparently a hydrated form of iron (iii)oxide. When copper metal is exposed to the environment, it combines with oxygen in. Copper Rust Formula.
From artyclick.com
Copper Rust Color ArtyClick Copper Rust Formula List some of the methods used to prevent or slow corrosion. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. Rust is apparently a hydrated form of iron (iii)oxide. Rust forms when metal alloys containing. Copper Rust Formula.
From blog.thepipingmart.com
303 Stainless Steel vs 188 Steel What's the Difference Copper Rust Formula To understand the process of corrosion. What is the chemical formula for corrosion in copper and silver?. Copper does not rust in water, but it can corrode. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. When exposed to water, especially if it contains oxygen and other corrosive. List some of the methods used to. Copper Rust Formula.
From giovani-khatfield.blogspot.com
What Is the Chemical Formula for Copper I Nitrite Copper Rust Formula What is the chemical formula for corrosion in copper and silver?. Corrosion is usually defined as the degradation of metals due to an. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. Rust forms when metal alloys containing iron undergo the. When copper metal is exposed to the environment, it combines with oxygen in the. Copper Rust Formula.
From dasetec.weebly.com
What is rust dasetec Copper Rust Formula Hint :metals when exposed to air or some chemicals for a long. Rust forms when metal alloys containing iron undergo the. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. When exposed to water,. Copper Rust Formula.
From www.slideshare.net
Corrosion, standard grade chemistry Copper Rust Formula To understand the process of corrosion. Copper does not rust in water, but it can corrode. List some of the methods used to prevent or slow corrosion. After a few years, this tarnish gradually changes to dark brown or. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Hint :metals when exposed to air or some. Copper Rust Formula.
From www.slideshare.net
Corrosion, standard grade chemistry Copper Rust Formula When exposed to water, especially if it contains oxygen and other corrosive. What is the chemical formula for corrosion in copper and silver?. After a few years, this tarnish gradually changes to dark brown or. Rust is apparently a hydrated form of iron (iii)oxide. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Corrosion is usually. Copper Rust Formula.
From saylordotorg.github.io
Corrosion Copper Rust Formula After a few years, this tarnish gradually changes to dark brown or. When exposed to water, especially if it contains oxygen and other corrosive. Copper does not rust in water, but it can corrode. Rust is apparently a hydrated form of iron (iii)oxide. When copper metal is exposed to the environment, it combines with oxygen in the air to generate. Copper Rust Formula.
From shapeguidance1.gitlab.io
Awesome Chemical Equation For The Formation Of Rust Anaerobic Respiration Copper Rust Formula Hint :metals when exposed to air or some chemicals for a long. When exposed to water, especially if it contains oxygen and other corrosive. Rust forms when metal alloys containing iron undergo the. To understand the process of corrosion. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu.. Copper Rust Formula.
From signalticket9.pythonanywhere.com
Fantastic Corrosion Of Copper Equation Rusting Chemical Copper Rust Formula After a few years, this tarnish gradually changes to dark brown or. Hint :metals when exposed to air or some chemicals for a long. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. List some of the methods used to prevent or slow corrosion. Corrosion is a galvanic. Copper Rust Formula.
From www.vrogue.co
Smart Speed Vs Time Graphs Chemical Equation For Rust vrogue.co Copper Rust Formula Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Hint :metals when exposed to air or some chemicals for a long. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu.. Copper Rust Formula.
From www.desertcart.in
Buy Flitz Brass and Copper Tarnish Remover, Powerful Formula That Copper Rust Formula Rust is apparently a hydrated form of iron (iii)oxide. Rust forms when metal alloys containing iron undergo the. After a few years, this tarnish gradually changes to dark brown or. Copper does not rust in water, but it can corrode. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide. Copper Rust Formula.
From icolorpalette.com
Copper Rust information Hsl Rgb Pantone Copper Rust Formula When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. To understand the process of corrosion. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. Copper does not rust in water, but it can corrode. Corrosion is usually defined as the degradation of metals. Copper Rust Formula.
From shipheat9.gitlab.io
Peerless What Is The Balanced Equation For Rust Effect Of Copper Rust Formula When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. After a few years, this tarnish gradually changes to dark brown or. Corrosion is usually defined as the degradation of metals due to an. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. Hint. Copper Rust Formula.
From www.vecteezy.com
Process of rusting chemical equation 1486308 Vector Art at Vecteezy Copper Rust Formula When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. What is the chemical formula for corrosion in copper and silver?. When exposed to water, especially if it contains oxygen and other corrosive. Corrosion is usually defined as the degradation of metals due to an. When exposed to the. Copper Rust Formula.
From www.dreamstime.com
Process of Rusting Chemical Equation Stock Vector Illustration of Copper Rust Formula Copper does not rust in water, but it can corrode. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. List some of the methods used to prevent or slow corrosion. Corrosion is usually defined as the degradation of metals due to an. What is the chemical formula for corrosion in copper and silver?. To understand the. Copper Rust Formula.
From animalia-life.club
Rusting Of Iron Chemical Reaction Copper Rust Formula List some of the methods used to prevent or slow corrosion. To understand the process of corrosion. Rust forms when metal alloys containing iron undergo the. Rust is apparently a hydrated form of iron (iii)oxide. After a few years, this tarnish gradually changes to dark brown or. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to. Copper Rust Formula.
From brainly.in
Chemical equation for the corrosion of copper... Brainly.in Copper Rust Formula After a few years, this tarnish gradually changes to dark brown or. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. Rust forms when metal alloys containing iron undergo the. Hint :metals when exposed to air or some chemicals for a long. List some of the methods used. Copper Rust Formula.
From www.walmart.com
Copper, RustOleum Universal All Surface Interior/Exterior Matte Copper Rust Formula When exposed to water, especially if it contains oxygen and other corrosive. Corrosion is usually defined as the degradation of metals due to an. What is the chemical formula for corrosion in copper and silver?. Rust is apparently a hydrated form of iron (iii)oxide. Rust forms when metal alloys containing iron undergo the. When exposed to the atmosphere, copper oxidizes,. Copper Rust Formula.
From ar.inspiredpencil.com
Chemical Equation Of Rusting Iron Copper Rust Formula After a few years, this tarnish gradually changes to dark brown or. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. Hint :metals when exposed to air or some chemicals for a long. Copper does not rust in water, but it can corrode. When exposed to water, especially. Copper Rust Formula.
From www.artofit.org
Creating a copper patina fast Artofit Copper Rust Formula Hint :metals when exposed to air or some chemicals for a long. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. Corrosion is usually defined as the degradation of metals due to an. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. When exposed. Copper Rust Formula.
From www.vecteezy.com
Process of rusting chemical equation 1868434 Vector Art at Vecteezy Copper Rust Formula To understand the process of corrosion. Rust forms when metal alloys containing iron undergo the. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Copper does not rust in water, but it can corrode. List some of the methods used to prevent or slow corrosion. Corrosion is usually defined as the degradation of metals due to. Copper Rust Formula.
From www.vectorstock.com
Process rusting chemical equation Royalty Free Vector Image Copper Rust Formula Hint :metals when exposed to air or some chemicals for a long. When exposed to water, especially if it contains oxygen and other corrosive. Rust forms when metal alloys containing iron undergo the. What is the chemical formula for corrosion in copper and silver?. Copper does not rust in water, but it can corrode. Corrosion is a galvanic process by. Copper Rust Formula.
From www.youtube.com
How to Realistic Looking Rust/Copper Patina/Faux Finishes YouTube Copper Rust Formula What is the chemical formula for corrosion in copper and silver?. When exposed to water, especially if it contains oxygen and other corrosive. Copper does not rust in water, but it can corrode. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. List some of the methods used to prevent or slow corrosion. Rust forms. Copper Rust Formula.
From brainly.in
What is the chemical formula of rust Brainly.in Copper Rust Formula Rust is apparently a hydrated form of iron (iii)oxide. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. When exposed to water, especially if it contains oxygen and other corrosive. When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. To understand the process of. Copper Rust Formula.
From ar.inspiredpencil.com
Chemical Changes Rust Copper Rust Formula When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. List some of the methods used to prevent or slow corrosion. When exposed to water, especially if it contains oxygen and other corrosive. Rust forms when metal alloys containing iron undergo the. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Rust. Copper Rust Formula.
From blog.thepipingmart.com
Can Copper Corrode? Copper Rust Formula When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Corrosion is usually defined as the degradation of metals due to an. To understand the process of corrosion. After a few years, this tarnish gradually changes to dark brown or. What is the. Copper Rust Formula.
From blog.thepipingmart.com
What is Copper Corrosion? An Overview Copper Rust Formula When copper metal is exposed to the environment, it combines with oxygen in the air to generate copper (i) oxide cu. Rust is apparently a hydrated form of iron (iii)oxide. Hint :metals when exposed to air or some chemicals for a long. Copper does not rust in water, but it can corrode. To understand the process of corrosion. What is. Copper Rust Formula.
From www.teachoo.com
Does Copper get Rusted? with Chemical Reaction Teachoo Copper Rust Formula To understand the process of corrosion. Rust forms when metal alloys containing iron undergo the. Corrosion is a galvanic process by which metals deteriorate through oxidation—usually but not. Rust is apparently a hydrated form of iron (iii)oxide. What is the chemical formula for corrosion in copper and silver?. When exposed to water, especially if it contains oxygen and other corrosive.. Copper Rust Formula.
From byjus.com
what is the formula of rusting of iron Copper Rust Formula After a few years, this tarnish gradually changes to dark brown or. Corrosion is usually defined as the degradation of metals due to an. Copper does not rust in water, but it can corrode. When exposed to water, especially if it contains oxygen and other corrosive. Rust forms when metal alloys containing iron undergo the. When copper metal is exposed. Copper Rust Formula.
From blog.thepipingmart.com
The Weight Formula for Steel Copper Rust Formula To understand the process of corrosion. List some of the methods used to prevent or slow corrosion. Corrosion is usually defined as the degradation of metals due to an. After a few years, this tarnish gradually changes to dark brown or. Rust is apparently a hydrated form of iron (iii)oxide. When exposed to the atmosphere, copper oxidizes, causing normally bright. Copper Rust Formula.
From blog.thepipingmart.com
How to DIY Copper Plate Steel for Rust Protection Copper Rust Formula Copper does not rust in water, but it can corrode. When exposed to the atmosphere, copper oxidizes, causing normally bright copper surfaces to tarnish. After a few years, this tarnish gradually changes to dark brown or. List some of the methods used to prevent or slow corrosion. Hint :metals when exposed to air or some chemicals for a long. What. Copper Rust Formula.