Rate Constant K Equation . The rateconstant (k) of a reaction can be calculated using: The initialrates and the rate equation. Determine the numerical value of the rate constant k with appropriate units. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The units for the rate of a reaction are mol/l/s. If the rate constant doubles, for example,.
from chemistryguru.com.sg
The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Determine the numerical value of the rate constant k with appropriate units. The units for the rate of a reaction are mol/l/s. If the rate constant doubles, for example,. The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using:
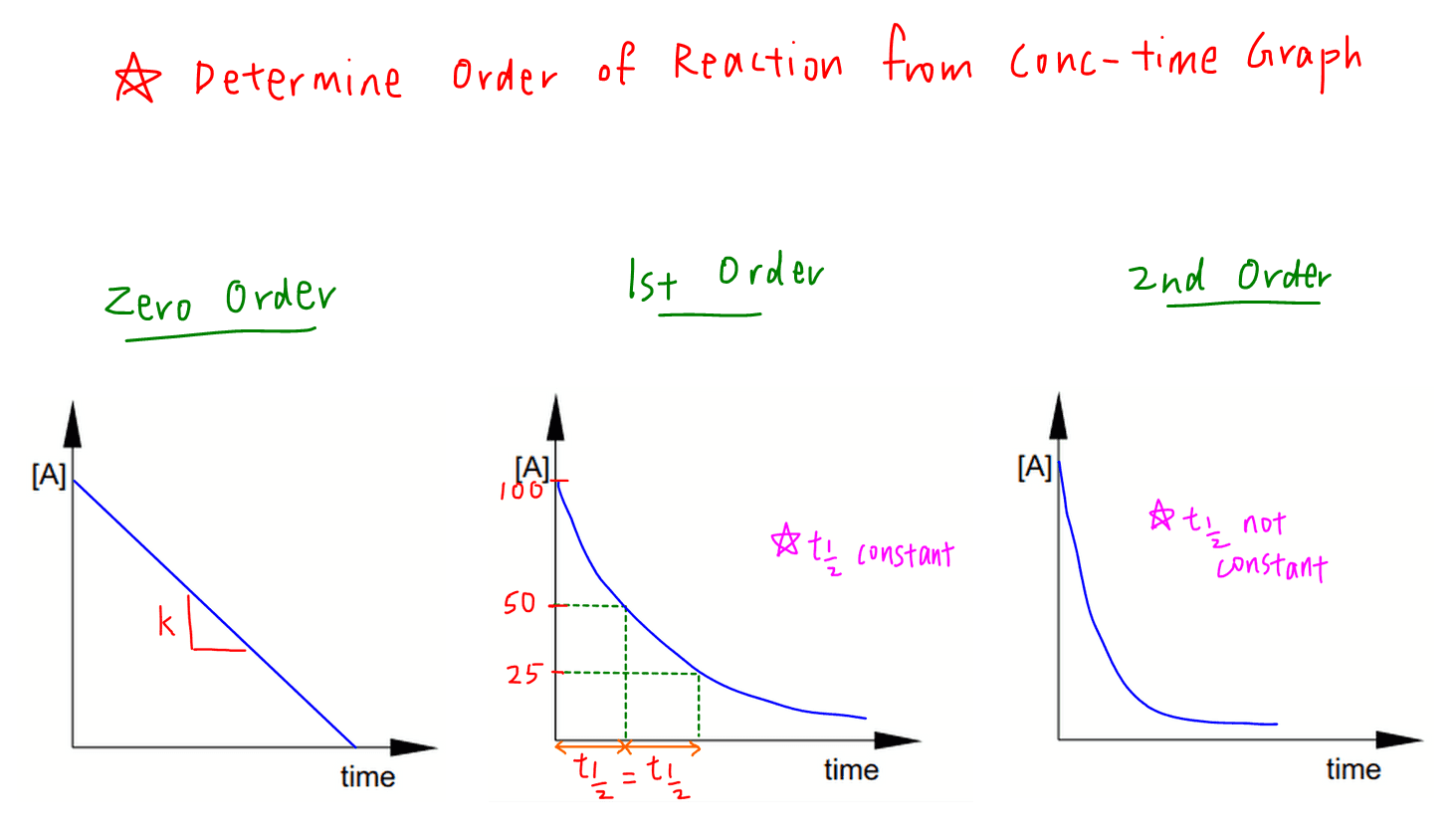
Rate Equation and Order of Reaction
Rate Constant K Equation If the rate constant doubles, for example,. The rateconstant (k) of a reaction can be calculated using: The units for the rate of a reaction are mol/l/s. Determine the numerical value of the rate constant k with appropriate units. The initialrates and the rate equation. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. If the rate constant doubles, for example,.
From general.chemistrysteps.com
Arrhenius Equation Chemistry Steps Rate Constant K Equation The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The initialrates and the rate equation. Determine the numerical value of the rate constant k with appropriate units. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. The units for. Rate Constant K Equation.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant K Equation Determine the numerical value of the rate constant k with appropriate units. The initialrates and the rate equation. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The units for the rate of a reaction are mol/l/s. The rateconstant (k) of a reaction can be calculated using:. Rate Constant K Equation.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Rate Constant K Equation If the rate constant doubles, for example,. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k) of a reaction can be calculated using: The initialrates and the rate equation. Determine the numerical value of the rate constant k with appropriate units. The units for. Rate Constant K Equation.
From www.sliderbase.com
Determining Order with Concentration vs. Time data Rate Constant K Equation Determine the numerical value of the rate constant k with appropriate units. If the rate constant doubles, for example,. The rateconstant (k) of a reaction can be calculated using: The units for the rate of a reaction are mol/l/s. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that. Rate Constant K Equation.
From general.chemistrysteps.com
Kp and Kc Relationship Chemistry Steps Rate Constant K Equation The initialrates and the rate equation. The units for the rate of a reaction are mol/l/s. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. Determine the numerical value of. Rate Constant K Equation.
From www.youtube.com
Integrated Rate Equation for third Order Reaction Third Order Rate Constant K Equation Determine the numerical value of the rate constant k with appropriate units. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. The units for the rate of a reaction are. Rate Constant K Equation.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant K Equation If the rate constant doubles, for example,. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k) of a reaction can be calculated using: The units for the rate of a reaction are mol/l/s. The initialrates and the rate equation. Determine the numerical value of. Rate Constant K Equation.
From www.sliderbase.com
Rate Laws Presentation Chemistry Rate Constant K Equation The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. The initialrates and the rate equation. Determine the numerical value of the rate constant k with appropriate units. The units for the rate of a reaction are mol/l/s. The rate constant, k, is a number that connects the concentration of reactants in a. Rate Constant K Equation.
From www.youtube.com
How to Determine Units of Rate Constant k (Shortcut with Examples Rate Constant K Equation The units for the rate of a reaction are mol/l/s. Determine the numerical value of the rate constant k with appropriate units. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. If the rate constant doubles, for example,. The initialrates and the rate equation. The rateconstant (k). Rate Constant K Equation.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Rate Constant K Equation Determine the numerical value of the rate constant k with appropriate units. The rateconstant (k) of a reaction can be calculated using: The units for the rate of a reaction are mol/l/s. If the rate constant doubles, for example,. The initialrates and the rate equation. The rate constant, k, is a number that connects the concentration of reactants in a. Rate Constant K Equation.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical Rate Constant K Equation The rateconstant (k) of a reaction can be calculated using: The units for the rate of a reaction are mol/l/s. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The initialrates and the rate equation. Determine the numerical value of the rate constant k with appropriate units.. Rate Constant K Equation.
From byjus.com
The rate constant of a reaction is 1.2×10^ 3sec^ 1 at 30℃and 2.1×10 Rate Constant K Equation If the rate constant doubles, for example,. The initialrates and the rate equation. Determine the numerical value of the rate constant k with appropriate units. The units for the rate of a reaction are mol/l/s. The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants in a. Rate Constant K Equation.
From byjus.com
What is the unit of rate constant for first order reaction Rate Constant K Equation The units for the rate of a reaction are mol/l/s. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. Determine the numerical value of the rate constant k with appropriate. Rate Constant K Equation.
From www.youtube.com
Rate equation and the units of the rate constant YouTube Rate Constant K Equation The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Determine the numerical value of the rate constant k with appropriate units. The initialrates and the rate equation. The units for the rate of a reaction are mol/l/s. The rateconstant (k) of a reaction can be calculated using:. Rate Constant K Equation.
From facts.net
14 Fascinating Facts About Rate Constant Rate Constant K Equation Determine the numerical value of the rate constant k with appropriate units. The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. If the rate constant doubles, for example,. The initialrates and the rate equation. The units for. Rate Constant K Equation.
From www.researchgate.net
Hi there, I have a problem regarding the reaction rate calculation Rate Constant K Equation If the rate constant doubles, for example,. The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The units for the rate of a reaction are mol/l/s. The initialrates and the rate equation. Determine the numerical value of. Rate Constant K Equation.
From www.toppr.com
Derive an integrated rate equation for rate constant of a zero order Rate Constant K Equation If the rate constant doubles, for example,. Determine the numerical value of the rate constant k with appropriate units. The units for the rate of a reaction are mol/l/s. The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants in a. Rate Constant K Equation.
From www.youtube.com
units of the rate constant k derivations YouTube Rate Constant K Equation The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The units for the rate of a reaction are mol/l/s. If the rate constant doubles, for example,. The rateconstant (k) of a reaction can be calculated using: The initialrates and the rate equation. Determine the numerical value of. Rate Constant K Equation.
From socratic.org
What is the halflife of a firstorder reaction with a rate constant of Rate Constant K Equation The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. If the rate constant doubles, for example,. Determine the numerical value of the rate constant k with appropriate units. The units for. Rate Constant K Equation.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant K Equation If the rate constant doubles, for example,. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The units for the rate of a reaction are mol/l/s. The rateconstant (k) of a reaction can be calculated using: The initialrates and the rate equation. Determine the numerical value of. Rate Constant K Equation.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Rate Constant K Equation The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k) of a reaction can be calculated using: The initialrates and the rate equation. If the rate constant doubles, for example,. Determine the numerical value of the rate constant k with appropriate units. The units for. Rate Constant K Equation.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Rate Constant K Equation The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. If the rate constant doubles, for example,. Determine the numerical value of the rate constant k with appropriate units. The units for the rate of a reaction are mol/l/s. The rateconstant (k) of a reaction can be calculated. Rate Constant K Equation.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Rate Constant K Equation The initialrates and the rate equation. The units for the rate of a reaction are mol/l/s. If the rate constant doubles, for example,. Determine the numerical value of the rate constant k with appropriate units. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k). Rate Constant K Equation.
From www.youtube.com
Temperature and Arrhenius equation YouTube Rate Constant K Equation The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Determine the numerical value of the rate constant k with appropriate units. The rateconstant (k) of a reaction can be calculated using: The initialrates and the rate equation. The units for the rate of a reaction are mol/l/s.. Rate Constant K Equation.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Rate Constant K Equation The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The initialrates and the rate equation. The units for the rate of a reaction are mol/l/s. If the rate constant doubles, for example,. Determine the numerical value of the rate constant k with appropriate units. The rateconstant (k). Rate Constant K Equation.
From byjus.com
According to Arrhenius equation rate constant k is equal to A · e E a Rate Constant K Equation The units for the rate of a reaction are mol/l/s. Determine the numerical value of the rate constant k with appropriate units. The initialrates and the rate equation. If the rate constant doubles, for example,. The rateconstant (k) of a reaction can be calculated using: The rate constant, k, is a number that connects the concentration of reactants in a. Rate Constant K Equation.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Rate Constant K Equation The initialrates and the rate equation. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Determine the numerical value of the rate constant k with appropriate units. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. The units for. Rate Constant K Equation.
From facts.net
13 Mindblowing Facts About Reaction Rate Constant Rate Constant K Equation The rateconstant (k) of a reaction can be calculated using: The units for the rate of a reaction are mol/l/s. The initialrates and the rate equation. If the rate constant doubles, for example,. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Determine the numerical value of. Rate Constant K Equation.
From www.slideshare.net
Chemical Rate Constant K Equation The initialrates and the rate equation. If the rate constant doubles, for example,. The units for the rate of a reaction are mol/l/s. Determine the numerical value of the rate constant k with appropriate units. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k). Rate Constant K Equation.
From pametno21.blogspot.com
K Constant Formula Chemistry pametno Rate Constant K Equation The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Determine the numerical value of the rate constant k with appropriate units. If the rate constant doubles, for example,. The initialrates and the rate equation. The units for the rate of a reaction are mol/l/s. The rateconstant (k). Rate Constant K Equation.
From www.chegg.com
Solved The temperature dependence of the reaction rate Rate Constant K Equation The units for the rate of a reaction are mol/l/s. The initialrates and the rate equation. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. Determine the numerical value of. Rate Constant K Equation.
From lavelle.chem.ucla.edu
halflife CHEMISTRY COMMUNITY Rate Constant K Equation The units for the rate of a reaction are mol/l/s. Determine the numerical value of the rate constant k with appropriate units. The initialrates and the rate equation. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. The rate constant, k, is a number that connects the concentration of reactants in a. Rate Constant K Equation.
From askfilo.com
57. The rate constant for the first order of N₂O5 is given Rate Constant K Equation The initialrates and the rate equation. If the rate constant doubles, for example,. Determine the numerical value of the rate constant k with appropriate units. The units for the rate of a reaction are mol/l/s. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rateconstant (k). Rate Constant K Equation.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant K Equation The initialrates and the rate equation. The units for the rate of a reaction are mol/l/s. Determine the numerical value of the rate constant k with appropriate units. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. If the rate constant doubles, for example,. The rateconstant (k). Rate Constant K Equation.
From www.vrogue.co
Solved Part B What Is The Rate Constant Of A First Or vrogue.co Rate Constant K Equation Determine the numerical value of the rate constant k with appropriate units. The rateconstant (k) of a reaction can be calculated using: If the rate constant doubles, for example,. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The initialrates and the rate equation. The units for. Rate Constant K Equation.