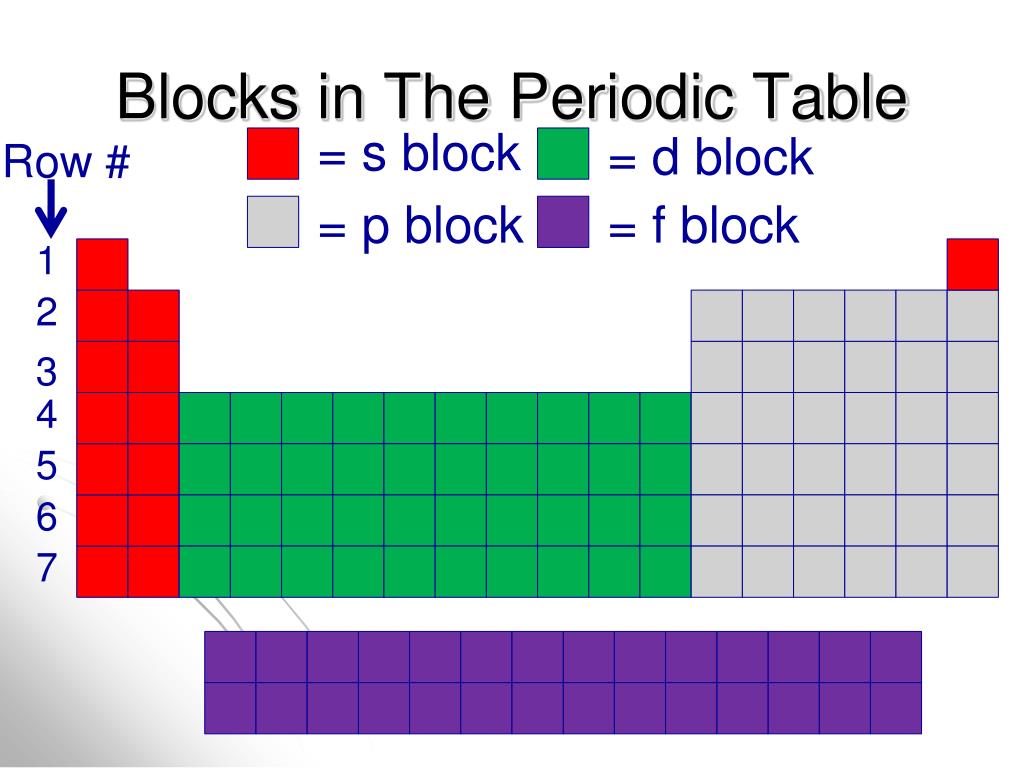
Energy Blocks Periodic Table . All the elements along a period have the same number of electron shells. Learn how to identify the block of an element on the periodic table. All the elements down a group have the same number of outer electrons, this number is indicated by the group number. Are electropositive and chemically active. All elements belong to one of four main blocks: Are either alkali metals or alkaline earth metals. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: Learn how to use the atomic number, electron configuration, and. Interactive periodic table showing names, electrons, and oxidation states. Each block indicates which electron sublevel is in the process of being filled. Are soft and have low melting points. Periodic table blocks are sets of elements grouped by their valence electron orbitals. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Visualize trends, 3d orbitals, isotopes, and mix compounds. The \(s\), \(p\), \(d\), and \(f\) blocks are.
from www.slideserve.com
Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: Interactive periodic table showing names, electrons, and oxidation states. All the elements along a period have the same number of electron shells. Are electropositive and chemically active. Learn how to identify the block of an element on the periodic table. Are either alkali metals or alkaline earth metals. Visualize trends, 3d orbitals, isotopes, and mix compounds. Are soft and have low melting points. All the elements down a group have the same number of outer electrons, this number is indicated by the group number. The \(s\), \(p\), \(d\), and \(f\) blocks are.
PPT Blocks in The Periodic Table PowerPoint Presentation, free
Energy Blocks Periodic Table Periodic table blocks are sets of elements grouped by their valence electron orbitals. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: All the elements down a group have the same number of outer electrons, this number is indicated by the group number. Each block indicates which electron sublevel is in the process of being filled. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Are either alkali metals or alkaline earth metals. Are electropositive and chemically active. Interactive periodic table showing names, electrons, and oxidation states. Periodic table blocks are sets of elements grouped by their valence electron orbitals. All elements belong to one of four main blocks: Visualize trends, 3d orbitals, isotopes, and mix compounds. Are soft and have low melting points. All the elements along a period have the same number of electron shells. Learn how to identify the block of an element on the periodic table. Learn how to use the atomic number, electron configuration, and. The \(s\), \(p\), \(d\), and \(f\) blocks are.
From www.pinterest.co.uk
This is a periodic table in which the elements are ordered by the Energy Blocks Periodic Table Visualize trends, 3d orbitals, isotopes, and mix compounds. Learn how to identify the block of an element on the periodic table. Are electropositive and chemically active. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Are soft and have low melting points. Periodic table blocks are sets. Energy Blocks Periodic Table.
From sciencenotes.org
Periodic Table Electron Configuration with Orbital Blocks Science Energy Blocks Periodic Table Periodic table blocks are sets of elements grouped by their valence electron orbitals. All the elements along a period have the same number of electron shells. Are soft and have low melting points. The \(s\), \(p\), \(d\), and \(f\) blocks are. All the elements down a group have the same number of outer electrons, this number is indicated by the. Energy Blocks Periodic Table.
From chem.libretexts.org
6.9 Electron Configurations and the Periodic Table Chemistry LibreTexts Energy Blocks Periodic Table Are soft and have low melting points. Visualize trends, 3d orbitals, isotopes, and mix compounds. All the elements along a period have the same number of electron shells. Are electropositive and chemically active. Learn how to identify the block of an element on the periodic table. Are either alkali metals or alkaline earth metals. Learn how to use the atomic. Energy Blocks Periodic Table.
From preparatorychemistry.com
Electron configurations Energy Blocks Periodic Table Learn how to identify the block of an element on the periodic table. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: All elements belong to one of four main blocks: Are soft and have low melting points. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel. Energy Blocks Periodic Table.
From utedzz.blogspot.com
Labeled Periodic Table Energy Levels Periodic Table Timeline Energy Blocks Periodic Table The \(s\), \(p\), \(d\), and \(f\) blocks are. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: Are soft and have low melting points. Are either alkali metals or alkaline earth metals. Learn how to use the atomic number, electron configuration, and. Periodic table blocks are sets of elements grouped by their valence. Energy Blocks Periodic Table.
From inspiritvr.com
Blocks in periodic table Study Guide Inspirit Energy Blocks Periodic Table Periodic table blocks are sets of elements grouped by their valence electron orbitals. All the elements down a group have the same number of outer electrons, this number is indicated by the group number. Are soft and have low melting points. All the elements along a period have the same number of electron shells. Each block indicates which electron sublevel. Energy Blocks Periodic Table.
From saylordotorg.github.io
Electronic Structure and the Periodic Table Energy Blocks Periodic Table Interactive periodic table showing names, electrons, and oxidation states. Are electropositive and chemically active. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. The \(s\), \(p\), \(d\), and \(f\) blocks are. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons:. Energy Blocks Periodic Table.
From chemwiki.ucdavis.edu
Electron Configuration of Transition Metals Chemwiki Energy Blocks Periodic Table Each block indicates which electron sublevel is in the process of being filled. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: All the elements along a period have the same number of electron shells. All elements belong to one of four main blocks: Learn how to identify the block of an element. Energy Blocks Periodic Table.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts Energy Blocks Periodic Table Are soft and have low melting points. Each block indicates which electron sublevel is in the process of being filled. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. The \(s\), \(p\), \(d\), and \(f\) blocks are. Learn how to identify the block of an element on. Energy Blocks Periodic Table.
From www.pinterest.pt
periodic table with ionization energies Google Search Ionization Energy Blocks Periodic Table Periodic table blocks are sets of elements grouped by their valence electron orbitals. Learn how to use the atomic number, electron configuration, and. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Learn how to identify the block of an element on the periodic table. Are electropositive. Energy Blocks Periodic Table.
From periodictableguide.com
Periodic table with Ionization Energy Values (Labeled Image) Energy Blocks Periodic Table Visualize trends, 3d orbitals, isotopes, and mix compounds. Are electropositive and chemically active. Periodic table blocks are sets of elements grouped by their valence electron orbitals. Learn how to identify the block of an element on the periodic table. Learn how to use the atomic number, electron configuration, and. Based on electron configurations, the periodic table can be divided into. Energy Blocks Periodic Table.
From www.ck12.org
A block diagram of the periodic table shows which sublevels are being Energy Blocks Periodic Table Are either alkali metals or alkaline earth metals. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: All the elements down a group have the same number of outer electrons, this number is indicated by the group number. Each block indicates which electron sublevel is in the process of being filled. Periodic table. Energy Blocks Periodic Table.
From www.periodictableprintable.com
Periodic Table With Electron Structure Of Energy Levels 2023 Periodic Energy Blocks Periodic Table All elements belong to one of four main blocks: Periodic table blocks are sets of elements grouped by their valence electron orbitals. Learn how to identify the block of an element on the periodic table. Interactive periodic table showing names, electrons, and oxidation states. Are soft and have low melting points. Are either alkali metals or alkaline earth metals. Are. Energy Blocks Periodic Table.
From utedzz.blogspot.com
Periodic Table Blocks S P D F Periodic Table Timeline Energy Blocks Periodic Table Learn how to use the atomic number, electron configuration, and. All the elements along a period have the same number of electron shells. Interactive periodic table showing names, electrons, and oxidation states. Periodic table blocks are sets of elements grouped by their valence electron orbitals. Are soft and have low melting points. Visualize trends, 3d orbitals, isotopes, and mix compounds.. Energy Blocks Periodic Table.
From www.slideserve.com
PPT Blocks in The Periodic Table PowerPoint Presentation, free Energy Blocks Periodic Table Learn how to use the atomic number, electron configuration, and. Visualize trends, 3d orbitals, isotopes, and mix compounds. Are electropositive and chemically active. The \(s\), \(p\), \(d\), and \(f\) blocks are. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: Interactive periodic table showing names, electrons, and oxidation states. Periodic table blocks are. Energy Blocks Periodic Table.
From www.youtube.com
Energy Levels & The Periodic Table YouTube Energy Blocks Periodic Table Periodic table blocks are sets of elements grouped by their valence electron orbitals. Are soft and have low melting points. The \(s\), \(p\), \(d\), and \(f\) blocks are. Are either alkali metals or alkaline earth metals. Visualize trends, 3d orbitals, isotopes, and mix compounds. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons:. Energy Blocks Periodic Table.
From chem.libretexts.org
5.5 Atomic Electron Configurations Chemistry LibreTexts Energy Blocks Periodic Table Visualize trends, 3d orbitals, isotopes, and mix compounds. Interactive periodic table showing names, electrons, and oxidation states. All the elements down a group have the same number of outer electrons, this number is indicated by the group number. The \(s\), \(p\), \(d\), and \(f\) blocks are. All the elements along a period have the same number of electron shells. All. Energy Blocks Periodic Table.
From periodictableguide.com
Periodic table with Ionization Energy Values (Labeled Image) Energy Blocks Periodic Table Are soft and have low melting points. Interactive periodic table showing names, electrons, and oxidation states. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Learn how to identify the block of an element on the periodic table. Element blocks are named for their characteristic orbital, which. Energy Blocks Periodic Table.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Energy Blocks Periodic Table Periodic table blocks are sets of elements grouped by their valence electron orbitals. All the elements down a group have the same number of outer electrons, this number is indicated by the group number. Learn how to use the atomic number, electron configuration, and. Are electropositive and chemically active. Based on electron configurations, the periodic table can be divided into. Energy Blocks Periodic Table.
From scientifictutor.org
Chem Complete Electron Configurations Scientific Tutor Energy Blocks Periodic Table Learn how to use the atomic number, electron configuration, and. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. All the elements down a group have the same number of outer electrons, this number is indicated by the group number. All elements belong to one of four. Energy Blocks Periodic Table.
From mavink.com
Periodic Table With Energy Levels Energy Blocks Periodic Table All the elements down a group have the same number of outer electrons, this number is indicated by the group number. All the elements along a period have the same number of electron shells. All elements belong to one of four main blocks: The \(s\), \(p\), \(d\), and \(f\) blocks are. Visualize trends, 3d orbitals, isotopes, and mix compounds. Are. Energy Blocks Periodic Table.
From www.teacharesources.com
Grade 9 The periodic table of the elements in animated PowerPoint Energy Blocks Periodic Table Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Interactive periodic table showing names, electrons, and oxidation states. The \(s\), \(p\), \(d\), and \(f\) blocks are. Visualize trends, 3d orbitals, isotopes, and mix compounds. Are either alkali metals or alkaline earth metals. All the elements down a. Energy Blocks Periodic Table.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Energy Blocks Periodic Table All the elements along a period have the same number of electron shells. Are either alkali metals or alkaline earth metals. All the elements down a group have the same number of outer electrons, this number is indicated by the group number. Periodic table blocks are sets of elements grouped by their valence electron orbitals. Are soft and have low. Energy Blocks Periodic Table.
From www.chegg.com
Solved Number the rows within each block of the periodic Energy Blocks Periodic Table Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Are soft and have low melting points. Learn how to use the atomic number, electron configuration, and. The \(s\), \(p\), \(d\), and \(f\) blocks are. Visualize trends, 3d orbitals, isotopes, and mix compounds. All the elements down a. Energy Blocks Periodic Table.
From saylordotorg.github.io
The Periodic Table and Periodic Trends Energy Blocks Periodic Table Learn how to use the atomic number, electron configuration, and. Periodic table blocks are sets of elements grouped by their valence electron orbitals. All elements belong to one of four main blocks: Learn how to identify the block of an element on the periodic table. Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3d orbitals, isotopes, and. Energy Blocks Periodic Table.
From www.slideserve.com
PPT The Periodic Table & Its Properties Chapters 5 & 6 PowerPoint Energy Blocks Periodic Table Interactive periodic table showing names, electrons, and oxidation states. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: Are either alkali metals or alkaline earth metals. Each block indicates which electron sublevel is in the process of being filled. Learn how to identify the block of an element on the periodic table. The. Energy Blocks Periodic Table.
From periodictableguide.com
Periodic table with Ionization Energy Values (Labeled Image) Energy Blocks Periodic Table Each block indicates which electron sublevel is in the process of being filled. All the elements down a group have the same number of outer electrons, this number is indicated by the group number. Periodic table blocks are sets of elements grouped by their valence electron orbitals. Are electropositive and chemically active. All elements belong to one of four main. Energy Blocks Periodic Table.
From insidechem.blogspot.com
Block periodic table INSIDE CHEMISTRY Energy Blocks Periodic Table All the elements along a period have the same number of electron shells. All elements belong to one of four main blocks: Visualize trends, 3d orbitals, isotopes, and mix compounds. Learn how to identify the block of an element on the periodic table. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in. Energy Blocks Periodic Table.
From pathwaystochemistry.com
Electron Configurations and Orbital Box Diagrams Pathways to Chemistry Energy Blocks Periodic Table All the elements along a period have the same number of electron shells. Learn how to identify the block of an element on the periodic table. Are electropositive and chemically active. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Interactive periodic table showing names, electrons, and. Energy Blocks Periodic Table.
From pediabay.com
Periodic Table & Energy Levels (Electrons per shell) Pediabay Energy Blocks Periodic Table Are soft and have low melting points. Are electropositive and chemically active. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: All elements belong to one of four main blocks: The \(s\), \(p\), \(d\), and \(f\) blocks are. Learn how to use the atomic number, electron configuration, and. Each block indicates which electron. Energy Blocks Periodic Table.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Energy Blocks Periodic Table Visualize trends, 3d orbitals, isotopes, and mix compounds. Learn how to identify the block of an element on the periodic table. All elements belong to one of four main blocks: Are soft and have low melting points. The \(s\), \(p\), \(d\), and \(f\) blocks are. Each block indicates which electron sublevel is in the process of being filled. All the. Energy Blocks Periodic Table.
From utedzz.blogspot.com
Periodic Table Energy Levels Periodic Table Timeline Energy Blocks Periodic Table Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Learn how to identify the block of an element on the periodic table. Are electropositive and chemically active. Are either alkali metals or alkaline earth metals. Are soft and have low melting points. Periodic table blocks are sets. Energy Blocks Periodic Table.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Energy Blocks Periodic Table Are soft and have low melting points. All the elements along a period have the same number of electron shells. All the elements down a group have the same number of outer electrons, this number is indicated by the group number. Interactive periodic table showing names, electrons, and oxidation states. Periodic table blocks are sets of elements grouped by their. Energy Blocks Periodic Table.
From utedzz.blogspot.com
Labeled Periodic Table Energy Levels Periodic Table Timeline Energy Blocks Periodic Table All the elements down a group have the same number of outer electrons, this number is indicated by the group number. Learn how to identify the block of an element on the periodic table. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: Each block indicates which electron sublevel is in the process. Energy Blocks Periodic Table.
From www.ck12.org
The Periodic Table and Electron Configurations CK12 Foundation Energy Blocks Periodic Table Periodic table blocks are sets of elements grouped by their valence electron orbitals. Interactive periodic table showing names, electrons, and oxidation states. The \(s\), \(p\), \(d\), and \(f\) blocks are. Are electropositive and chemically active. Learn how to use the atomic number, electron configuration, and. All the elements down a group have the same number of outer electrons, this number. Energy Blocks Periodic Table.