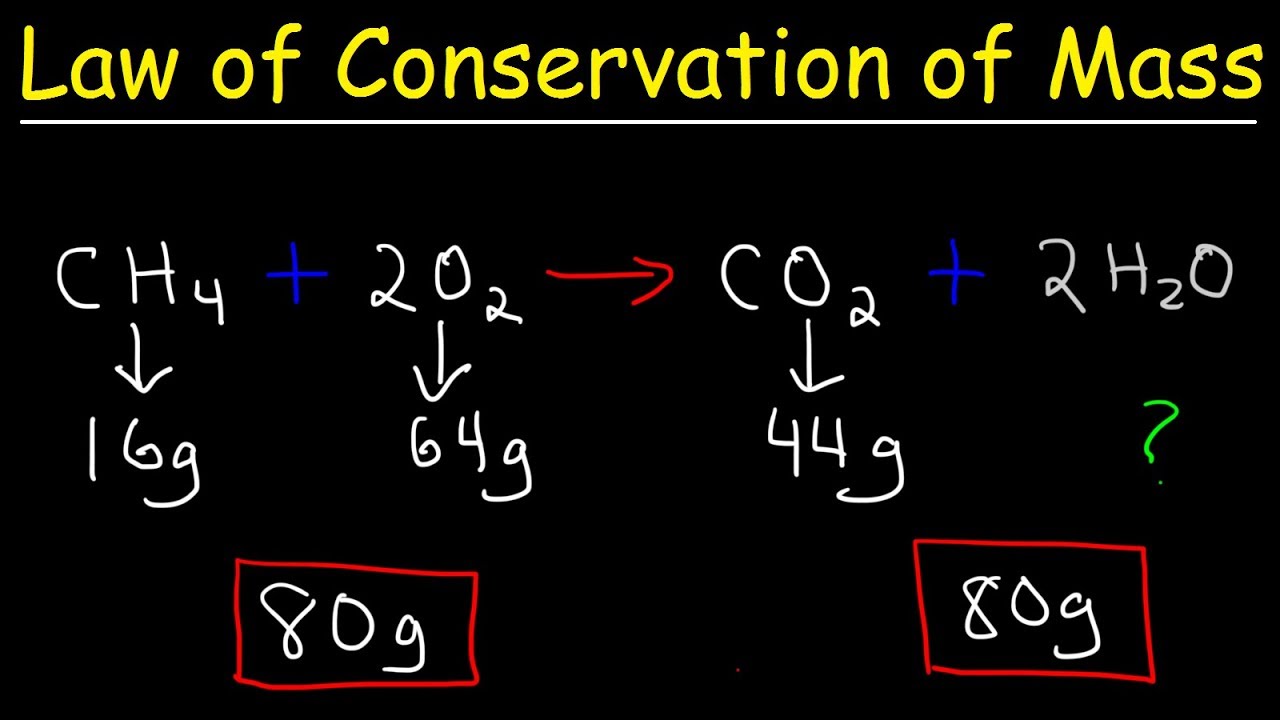
How To Find The Mass Of A Product In Chemistry . Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. The mass of the reactants. With this we can use the difference. To find the mass of a product, first convert the mass of the given reactant to moles. The molar mass of the reactants. Steps in converting between masses of reactant and product. To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. It can be calculated using: The relative formula mass of. This video covers how to find the mass of the products of a chemical reaction, an important. Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. To find the mass of products formed in a reaction the following pieces of information is needed: Then, use the mole ratio from the balanced chemical.
from orvelleblog.blogspot.com
The molar mass of the reactants. To find the mass of a product, first convert the mass of the given reactant to moles. It can be calculated using: The mass of the reactants. Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. The relative formula mass of. Then, use the mole ratio from the balanced chemical. Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. Steps in converting between masses of reactant and product.
Spice of Lyfe Chemical Equation Law Of Conservation Of Mass
How To Find The Mass Of A Product In Chemistry To find the mass of a product, first convert the mass of the given reactant to moles. It can be calculated using: With this we can use the difference. Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. To find the mass of products formed in a reaction the following pieces of information is needed: The relative formula mass of. To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. To find the mass of a product, first convert the mass of the given reactant to moles. The mass of the reactants. This video covers how to find the mass of the products of a chemical reaction, an important. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. Then, use the mole ratio from the balanced chemical. The molar mass of the reactants. Steps in converting between masses of reactant and product. Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations.
From www.teachoo.com
Law of Conservation of Mass Statement, Experiment, Examples (and mor How To Find The Mass Of A Product In Chemistry With this we can use the difference. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. This video covers how to find the mass of the products of a chemical reaction, an important. It can be calculated using: Convert the mass of one substance (substance a) to the corresponding. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
How to Calculate Mass Percent of Element in Compound Examples, Practice How To Find The Mass Of A Product In Chemistry To find the mass of a product, first convert the mass of the given reactant to moles. It can be calculated using: To find the mass of products formed in a reaction the following pieces of information is needed: To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. The mass of the. How To Find The Mass Of A Product In Chemistry.
From study.com
How to Find Mass Percent from Chemical Formulae Chemistry How To Find The Mass Of A Product In Chemistry Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. Steps in converting between masses of reactant and product. To find the mass of a product, first convert the mass of the. How To Find The Mass Of A Product In Chemistry.
From quizzlistoutmantle.z21.web.core.windows.net
How To Calculate Limiting And Excess Reactant How To Find The Mass Of A Product In Chemistry The mass of the reactants. To find the mass of a product, first convert the mass of the given reactant to moles. This video covers how to find the mass of the products of a chemical reaction, an important. Then, use the mole ratio from the balanced chemical. To perform a stoichiometric calculation, enter an equation of a chemical reaction. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
CHEM 101 Dimensional Analysis Stoichiometry and mass to mass How To Find The Mass Of A Product In Chemistry With this we can use the difference. The mass of the reactants. The molar mass of the reactants. Steps in converting between masses of reactant and product. Then, use the mole ratio from the balanced chemical. Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. To perform a. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
Calculations of reacting masses from chemical equations. YouTube How To Find The Mass Of A Product In Chemistry This video covers how to find the mass of the products of a chemical reaction, an important. Then, use the mole ratio from the balanced chemical. To find the mass of products formed in a reaction the following pieces of information is needed: It can be calculated using: To perform a stoichiometric calculation, enter an equation of a chemical reaction. How To Find The Mass Of A Product In Chemistry.
From lessonschoolchloroform.z21.web.core.windows.net
How To Work Out The Limiting Reactant How To Find The Mass Of A Product In Chemistry Steps in converting between masses of reactant and product. The relative formula mass of. Then, use the mole ratio from the balanced chemical. It can be calculated using: Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. The molar mass of the reactants. The mass of the reactants. To. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
[Example] How to determine the mass of products from the mass of How To Find The Mass Of A Product In Chemistry To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. With this we can use the difference. To find the mass of products formed in a reaction the following pieces of information is needed: The mass of the reactants. Using the law of conservation, we know that the mass before a reaction must. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
Mass Percent of a Solution Made Easy How to Calculate Mass or Make a How To Find The Mass Of A Product In Chemistry This video covers how to find the mass of the products of a chemical reaction, an important. It can be calculated using: Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. With this we can use the difference. To find the mass of a product, first convert the. How To Find The Mass Of A Product In Chemistry.
From www.goodscience.com.au
Balancing Chemical Equations Good Science How To Find The Mass Of A Product In Chemistry Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. This video covers how to find the mass of the products of a chemical reaction, an important. It can be calculated using: Convert the mass of one substance (substance a) to the corresponding number of moles using its molar. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
Chemistry Practice Problems Atomic Mass Calculations II YouTube How To Find The Mass Of A Product In Chemistry Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. It can be calculated using: With this we can use the difference. To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. The molar mass of the reactants. Convert the mass of. How To Find The Mass Of A Product In Chemistry.
From ar.inspiredpencil.com
Average Atomic Mass Periodic Table How To Find The Mass Of A Product In Chemistry It can be calculated using: Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. To find the mass of a product, first convert the mass of the given reactant to moles. With this we can use the difference. The relative formula mass of. To find the mass of products formed in. How To Find The Mass Of A Product In Chemistry.
From sciencenotes.org
Molar Mass and How to Find It How To Find The Mass Of A Product In Chemistry To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. The mass of the reactants. This video covers how to find the mass of the products of a chemical reaction, an important.. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
How To Measure Loss Of Mass In A Reaction Chemical Calculations How To Find The Mass Of A Product In Chemistry Then, use the mole ratio from the balanced chemical. The molar mass of the reactants. The relative formula mass of. The mass of the reactants. This video covers how to find the mass of the products of a chemical reaction, an important. To find the mass of products formed in a reaction the following pieces of information is needed: Learn. How To Find The Mass Of A Product In Chemistry.
From www.expii.com
Converting Mass of Reactant to Mass of Product — Examples Expii How To Find The Mass Of A Product In Chemistry To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. Steps in converting between masses of reactant and product. Convert the mass of one substance (substance a) to the corresponding number of moles. How To Find The Mass Of A Product In Chemistry.
From study.com
Calculating Amounts of Reactant from Amounts of Product Chemistry How To Find The Mass Of A Product In Chemistry With this we can use the difference. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. The molar mass of the reactants. To find the mass of a product, first convert the. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
Chemical Calculations Finding Mass of Products Given Mass of Reactants How To Find The Mass Of A Product In Chemistry Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. This video covers how to find the mass of the products of a chemical reaction, an important. To find the mass of a product, first convert the mass of the given reactant to moles. Then, use the mole ratio. How To Find The Mass Of A Product In Chemistry.
From www.pinterest.pt
Law of Conservation of Mass infographic diagram showing a lab How To Find The Mass Of A Product In Chemistry To find the mass of a product, first convert the mass of the given reactant to moles. To find the mass of products formed in a reaction the following pieces of information is needed: Then, use the mole ratio from the balanced chemical. Steps in converting between masses of reactant and product. Convert the mass of one substance (substance a). How To Find The Mass Of A Product In Chemistry.
From www.madebyteachers.com
Calculating Density, Mass, and Volume A Science worksheet Made By How To Find The Mass Of A Product In Chemistry With this we can use the difference. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. To find the mass of a product, first convert the mass of. How To Find The Mass Of A Product In Chemistry.
From rechschem.weebly.com
Percent Composition How To Find The Mass Of A Product In Chemistry Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. It can be calculated using: With this we can use the difference. The molar mass of the reactants. Steps in converting between masses of reactant and product. To find the mass of a product, first convert the mass of. How To Find The Mass Of A Product In Chemistry.
From materialcampusmatthews.z21.web.core.windows.net
Percent Yield And Theoretical Yield How To Find The Mass Of A Product In Chemistry This video covers how to find the mass of the products of a chemical reaction, an important. Steps in converting between masses of reactant and product. The mass of the reactants. The molar mass of the reactants. The relative formula mass of. Using the law of conservation, we know that the mass before a reaction must equal the mass after. How To Find The Mass Of A Product In Chemistry.
From www.expii.com
Converting Mass of Reactant to Mass of Product — Examples Expii How To Find The Mass Of A Product In Chemistry The relative formula mass of. To find the mass of a product, first convert the mass of the given reactant to moles. Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. Steps in converting between masses of reactant and product. To perform a stoichiometric calculation, enter an equation of a chemical. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
Calculate Mass of Product Formed (Example) YouTube How To Find The Mass Of A Product In Chemistry With this we can use the difference. The mass of the reactants. Steps in converting between masses of reactant and product. It can be calculated using: This video covers how to find the mass of the products of a chemical reaction, an important. The molar mass of the reactants. Learn how to calculate reacting masses and balance equations for igcse. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
Calculating Mass of Product in Chemical Reactions YouTube How To Find The Mass Of A Product In Chemistry To find the mass of products formed in a reaction the following pieces of information is needed: With this we can use the difference. This video covers how to find the mass of the products of a chemical reaction, an important. Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. Then,. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
Chemistry Calculation 3(mass of products) YouTube How To Find The Mass Of A Product In Chemistry Then, use the mole ratio from the balanced chemical. The mass of the reactants. The relative formula mass of. To find the mass of products formed in a reaction the following pieces of information is needed: It can be calculated using: This video covers how to find the mass of the products of a chemical reaction, an important. Using the. How To Find The Mass Of A Product In Chemistry.
From felishaleeber1999.blogspot.com
Felisha Leeber How To Find Mass From Volume Chemistry How To Find The Mass Of A Product In Chemistry With this we can use the difference. The mass of the reactants. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. This video covers how to find the mass of the products of a chemical reaction, an important. Convert the mass of one substance (substance a) to the corresponding. How To Find The Mass Of A Product In Chemistry.
From lessondbhypersonic.z21.web.core.windows.net
How To Do Percent Composition How To Find The Mass Of A Product In Chemistry Then, use the mole ratio from the balanced chemical. Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. With this we can use the difference. To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. Steps in converting between masses of. How To Find The Mass Of A Product In Chemistry.
From brooklynpio.blogspot.com
20+ Mass Percent Calculator Chemistry BrooklynPio How To Find The Mass Of A Product In Chemistry Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. To find the mass of products formed in a reaction the following pieces of information is needed: With this we can use. How To Find The Mass Of A Product In Chemistry.
From haipernews.com
How To Calculate Percentage Yield Haiper How To Find The Mass Of A Product In Chemistry The molar mass of the reactants. This video covers how to find the mass of the products of a chemical reaction, an important. Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. The mass of the reactants. Convert the mass of one substance (substance a) to the corresponding. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
Chemistry Calculation 2 (mass of products) YouTube How To Find The Mass Of A Product In Chemistry The relative formula mass of. The molar mass of the reactants. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. Steps in converting between masses of reactant and product. With this we can use the difference. Convert the mass of one substance (substance a) to the corresponding number of. How To Find The Mass Of A Product In Chemistry.
From printablezonenurdle.z13.web.core.windows.net
Limiting Reagent And Percent Yield Practice How To Find The Mass Of A Product In Chemistry Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. Then, use the mole ratio from the balanced chemical. To find the mass of products formed in a reaction the following pieces of information is needed: To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start. How To Find The Mass Of A Product In Chemistry.
From www.youtube.com
How to Calculate Mass Needed to Make a Solution YouTube How To Find The Mass Of A Product In Chemistry To find the mass of a product, first convert the mass of the given reactant to moles. Then, use the mole ratio from the balanced chemical. Convert the mass of one substance (substance a) to the corresponding number of moles using its molar mass. To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start. How To Find The Mass Of A Product In Chemistry.
From www.pinterest.fr
How to Calculate Percent Yield in Chemistry Teaching chemistry How To Find The Mass Of A Product In Chemistry This video covers how to find the mass of the products of a chemical reaction, an important. Learn how to calculate reacting masses and balance equations for igcse chemistry, using worked examples and examiner tips for accurate calculations. Using the law of conservation, we know that the mass before a reaction must equal the mass after a reaction. To find. How To Find The Mass Of A Product In Chemistry.
From www.chemicalslearning.com
Types of Reactants in Chemistry How To Find The Mass Of A Product In Chemistry The relative formula mass of. To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the start button. With this we can use the difference. This video covers how to find the mass of the products of a chemical reaction, an important. The molar mass of the reactants. To find the mass of a product, first. How To Find The Mass Of A Product In Chemistry.
From orvelleblog.blogspot.com
Spice of Lyfe Chemical Equation Law Of Conservation Of Mass How To Find The Mass Of A Product In Chemistry To find the mass of products formed in a reaction the following pieces of information is needed: The molar mass of the reactants. Steps in converting between masses of reactant and product. Then, use the mole ratio from the balanced chemical. To find the mass of a product, first convert the mass of the given reactant to moles. Convert the. How To Find The Mass Of A Product In Chemistry.