Fuel Balanced Chemical Reaction . Recognize a reaction as a combustion reaction. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Its molecular mass is 28.96 kg/kmol. Balance any equation or reaction using this chemical equation balancer! This is done here to simplify the calculations of. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Complete and balance chemical equations for combustion reactions. A complete combustion is a. Most familiar fuels are hydrocarbons and are denoted by the general. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. In this text, the reactions are balanced using 1 mol of fuel. Find out what type of reaction occured. Any material that can be burned to release thermal energy.
from www.windward.solutions
Find out what type of reaction occured. Any material that can be burned to release thermal energy. Complete and balance chemical equations for combustion reactions. This is done here to simplify the calculations of. Recognize a reaction as a combustion reaction. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. A complete combustion is a. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Balance any equation or reaction using this chemical equation balancer! Its molecular mass is 28.96 kg/kmol.
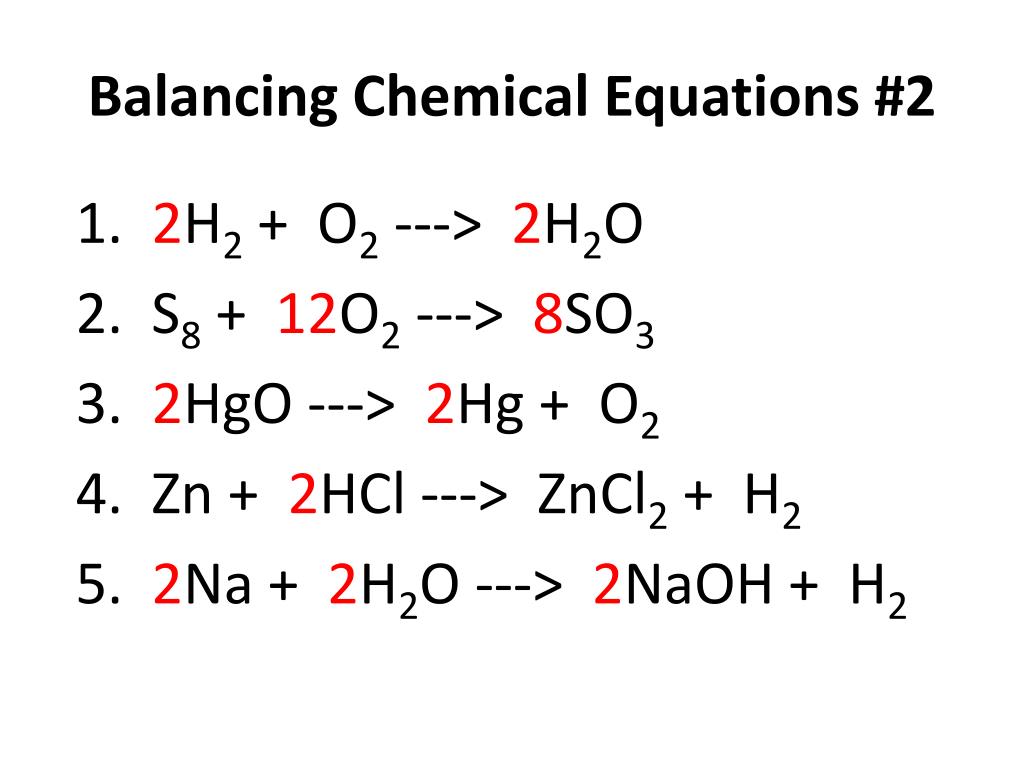
Balancing chemical equations kahoot
Fuel Balanced Chemical Reaction In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. This is done here to simplify the calculations of. A complete combustion is a. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Its molecular mass is 28.96 kg/kmol. Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and. Find out what type of reaction occured. Complete and balance chemical equations for combustion reactions. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. Balance any equation or reaction using this chemical equation balancer! Recognize a reaction as a combustion reaction. Most familiar fuels are hydrocarbons and are denoted by the general. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. In this text, the reactions are balanced using 1 mol of fuel. Any material that can be burned to release thermal energy.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID Fuel Balanced Chemical Reaction In this text, the reactions are balanced using 1 mol of fuel. Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and. Its molecular mass is 28.96 kg/kmol. Find out what type of reaction occured. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic. Fuel Balanced Chemical Reaction.
From www.youtube.com
Balancing Chemical Reactions (Guideline) YouTube Fuel Balanced Chemical Reaction Recognize a reaction as a combustion reaction. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Find out what type of reaction occured. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. This is done here to simplify the calculations of. A complete combustion is. Fuel Balanced Chemical Reaction.
From www.numerade.com
SOLVED Write an atom balanced chemical reaction equation for the Fuel Balanced Chemical Reaction Balance any equation or reaction using this chemical equation balancer! Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. Find out what type of reaction occured. Recognize a reaction as a combustion reaction. Any material that can be burned to release thermal energy. Its molecular mass is 28.96 kg/kmol. Good signs. Fuel Balanced Chemical Reaction.
From www.dreamstime.com
Fuel Combustion Process. Different Types of Fuel. Vector Illustration Fuel Balanced Chemical Reaction In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Find out what type of reaction occured. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. This is done here to simplify the calculations of. In this text, the reactions. Fuel Balanced Chemical Reaction.
From www.syngaschem.com
Synthesis Gas Chemistry and Synthetic Fuels Syngaschem BV Fuel Balanced Chemical Reaction Complete and balance chemical equations for combustion reactions. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Its molecular mass is 28.96 kg/kmol. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h,. Fuel Balanced Chemical Reaction.
From educationnerved.z4.web.core.windows.net
How To Write Combustion Reaction Equations Fuel Balanced Chemical Reaction Complete and balance chemical equations for combustion reactions. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Any material that can be burned to release thermal energy. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Recognize a reaction as a combustion reaction.. Fuel Balanced Chemical Reaction.
From www.chegg.com
Solved Write down balanced chemical reactions assuming Fuel Balanced Chemical Reaction Balance any equation or reaction using this chemical equation balancer! This is done here to simplify the calculations of. A complete combustion is a. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. Its molecular mass is 28.96 kg/kmol. Find out what type of reaction occured. Any material that can be. Fuel Balanced Chemical Reaction.
From www.wikihow.com
How to Balance Chemical Equations 11 Steps (with Pictures) Fuel Balanced Chemical Reaction A complete combustion is a. In this text, the reactions are balanced using 1 mol of fuel. Any material that can be burned to release thermal energy. Its molecular mass is 28.96 kg/kmol. Most familiar fuels are hydrocarbons and are denoted by the general. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Balance any. Fuel Balanced Chemical Reaction.
From www.youtube.com
Fossil Fuels IB Physics YouTube Fuel Balanced Chemical Reaction In this text, the reactions are balanced using 1 mol of fuel. Recognize a reaction as a combustion reaction. This is done here to simplify the calculations of. Most familiar fuels are hydrocarbons and are denoted by the general. Balance any equation or reaction using this chemical equation balancer! Omni's combustion reaction calculator will instantly balance any combustion reaction involving. Fuel Balanced Chemical Reaction.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision Fuel Balanced Chemical Reaction Any material that can be burned to release thermal energy. Find out what type of reaction occured. Complete and balance chemical equations for combustion reactions. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and. Fuel Balanced Chemical Reaction.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions Fuel Balanced Chemical Reaction Complete and balance chemical equations for combustion reactions. In this text, the reactions are balanced using 1 mol of fuel. Most familiar fuels are hydrocarbons and are denoted by the general. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. It usually occurs when a hydrocarbon reacts with. Fuel Balanced Chemical Reaction.
From revisechemistry.uk
Carbon Compounds as Fuels and Feedstock AQA C7 revisechemistry.uk Fuel Balanced Chemical Reaction Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. In this text, the reactions are balanced using 1 mol of fuel. Most familiar fuels are hydrocarbons and are denoted by the general. It usually. Fuel Balanced Chemical Reaction.
From www.scibond.com
How to Balance a Chemical Equations? SciBond Fuel Balanced Chemical Reaction In this text, the reactions are balanced using 1 mol of fuel. Recognize a reaction as a combustion reaction. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Find out what type of reaction occured. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or. Fuel Balanced Chemical Reaction.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Balanced Chemical Reaction Complete and balance chemical equations for combustion reactions. This is done here to simplify the calculations of. Any material that can be burned to release thermal energy. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Good signs that you're dealing with a combustion reaction include the presence. Fuel Balanced Chemical Reaction.
From www.mdpi.com
Applied Sciences Free FullText Physical and Chemical Features of Fuel Balanced Chemical Reaction Any material that can be burned to release thermal energy. This is done here to simplify the calculations of. A complete combustion is a. Recognize a reaction as a combustion reaction. Complete and balance chemical equations for combustion reactions. In this text, the reactions are balanced using 1 mol of fuel. Balance any equation or reaction using this chemical equation. Fuel Balanced Chemical Reaction.
From passmyexams.co.uk
Combustion of Alcohols Easy exam revision notes for GSCE Chemistry Fuel Balanced Chemical Reaction This is done here to simplify the calculations of. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and. Complete and balance chemical equations for combustion reactions. Its molecular mass is 28.96 kg/kmol. In this text, the reactions. Fuel Balanced Chemical Reaction.
From www.numerade.com
SOLVEDChem IA revicw Ethanol (CIOH) increasingly being used gasoline Fuel Balanced Chemical Reaction In this text, the reactions are balanced using 1 mol of fuel. Its molecular mass is 28.96 kg/kmol. Recognize a reaction as a combustion reaction. This is done here to simplify the calculations of. Most familiar fuels are hydrocarbons and are denoted by the general. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h,. Fuel Balanced Chemical Reaction.
From sciencenotes.org
Combustion Reaction Definition and Examples Fuel Balanced Chemical Reaction It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Most familiar fuels are hydrocarbons and are denoted by the general. Good signs that you're dealing with a combustion reaction include the presence of. Fuel Balanced Chemical Reaction.
From www.chegg.com
Solved Write down balanced chemical reactions assuming Fuel Balanced Chemical Reaction Balance any equation or reaction using this chemical equation balancer! Complete and balance chemical equations for combustion reactions. A complete combustion is a. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. This is done here to simplify the calculations of. Find out what type of reaction occured.. Fuel Balanced Chemical Reaction.
From www.youtube.com
Balanced Equation for the Combustion of Methane (CH4) YouTube Fuel Balanced Chemical Reaction Its molecular mass is 28.96 kg/kmol. Find out what type of reaction occured. Most familiar fuels are hydrocarbons and are denoted by the general. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Balance any equation or reaction using this chemical equation balancer! A complete combustion is a.. Fuel Balanced Chemical Reaction.
From www.tessshebaylo.com
Balanced Chemical Equation For The Complete Combustion Of Gasoline Fuel Balanced Chemical Reaction Balance any equation or reaction using this chemical equation balancer! Complete and balance chemical equations for combustion reactions. Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and. Its molecular mass is 28.96 kg/kmol. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Find out what. Fuel Balanced Chemical Reaction.
From www.slideshare.net
Organic chemistry fossil fuels Fuel Balanced Chemical Reaction This is done here to simplify the calculations of. Its molecular mass is 28.96 kg/kmol. Find out what type of reaction occured. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. In this text, the reactions are balanced using 1 mol of fuel. Any material that can be. Fuel Balanced Chemical Reaction.
From www.numerade.com
SOLVEDWrite the balanced chemical equation for the complete combustion Fuel Balanced Chemical Reaction In this text, the reactions are balanced using 1 mol of fuel. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Balance any equation or reaction using this chemical equation balancer! Complete and balance chemical equations for combustion reactions. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Omni's. Fuel Balanced Chemical Reaction.
From organicvsa.weebly.com
Combustion equations 4 chemical equations and stoichiometry organicvsa Fuel Balanced Chemical Reaction Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. Most familiar fuels are hydrocarbons and are denoted by the general. In this text, the reactions are balanced using 1 mol of fuel. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Complete and balance chemical. Fuel Balanced Chemical Reaction.
From www.numerade.com
SOLVED Write the combusi stion reactions for the burning of liquid Fuel Balanced Chemical Reaction Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Most familiar fuels are hydrocarbons and are denoted by the general. Find out what type of reaction occured. A complete combustion is a. Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and. It usually occurs when a. Fuel Balanced Chemical Reaction.
From www.windward.solutions
Balancing chemical equations kahoot Fuel Balanced Chemical Reaction Complete and balance chemical equations for combustion reactions. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Balance any equation or reaction using this chemical equation balancer! Any material that can be burned to release thermal energy. Stoichiometric or theoretical combustion is the ideal combustion process where fuel. Fuel Balanced Chemical Reaction.
From slidetodoc.com
Combustion Reactions Combustion is a chemical reaction where Fuel Balanced Chemical Reaction Balance any equation or reaction using this chemical equation balancer! This is done here to simplify the calculations of. In this text, the reactions are balanced using 1 mol of fuel. Any material that can be burned to release thermal energy. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Good signs that you're dealing. Fuel Balanced Chemical Reaction.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel Balanced Chemical Reaction Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Complete and balance chemical equations for combustion reactions. This is done here to simplify the calculations of. A complete combustion is. Fuel Balanced Chemical Reaction.
From studylib.net
COMBUSTION of PROPANE Fuel Balanced Chemical Reaction Find out what type of reaction occured. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Balance any equation or reaction using this chemical equation balancer! Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and. It usually occurs when. Fuel Balanced Chemical Reaction.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Fuel Balanced Chemical Reaction Find out what type of reaction occured. A complete combustion is a. Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Balance any equation or reaction using this chemical equation. Fuel Balanced Chemical Reaction.
From www.youtube.com
OCR C6 Fuel Cells (Higher) YouTube Fuel Balanced Chemical Reaction Most familiar fuels are hydrocarbons and are denoted by the general. Balance any equation or reaction using this chemical equation balancer! Its molecular mass is 28.96 kg/kmol. This is done here to simplify the calculations of. Recognize a reaction as a combustion reaction. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. In the. Fuel Balanced Chemical Reaction.
From saylordotorg.github.io
Applications of Redox Reactions Voltaic Cells Fuel Balanced Chemical Reaction Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. A complete combustion is a. Balance any equation or reaction using this chemical equation balancer! In this text, the reactions are. Fuel Balanced Chemical Reaction.
From www.wikihow.com
How to Balance Chemical Equations 11 Steps (with Pictures) Fuel Balanced Chemical Reaction In this text, the reactions are balanced using 1 mol of fuel. Recognize a reaction as a combustion reaction. Complete and balance chemical equations for combustion reactions. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Find out what type. Fuel Balanced Chemical Reaction.
From www.chegg.com
Solved 1. Write a balanced chemical equation, including Fuel Balanced Chemical Reaction Balance any equation or reaction using this chemical equation balancer! Omni's combustion reaction calculator will instantly balance any combustion reaction involving hydrocarbon or c, h, o organic substances. In this text, the reactions are balanced using 1 mol of fuel. Its molecular mass is 28.96 kg/kmol. In the most general sense, combustion involves a reaction between any combustible material and. Fuel Balanced Chemical Reaction.
From www.youtube.com
Balancing combustion reaction equation Gasoline Chemistry YouTube Fuel Balanced Chemical Reaction Its molecular mass is 28.96 kg/kmol. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. In the most general sense, combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product. Any material that can be burned to release thermal energy. This is done here to simplify the calculations. Fuel Balanced Chemical Reaction.