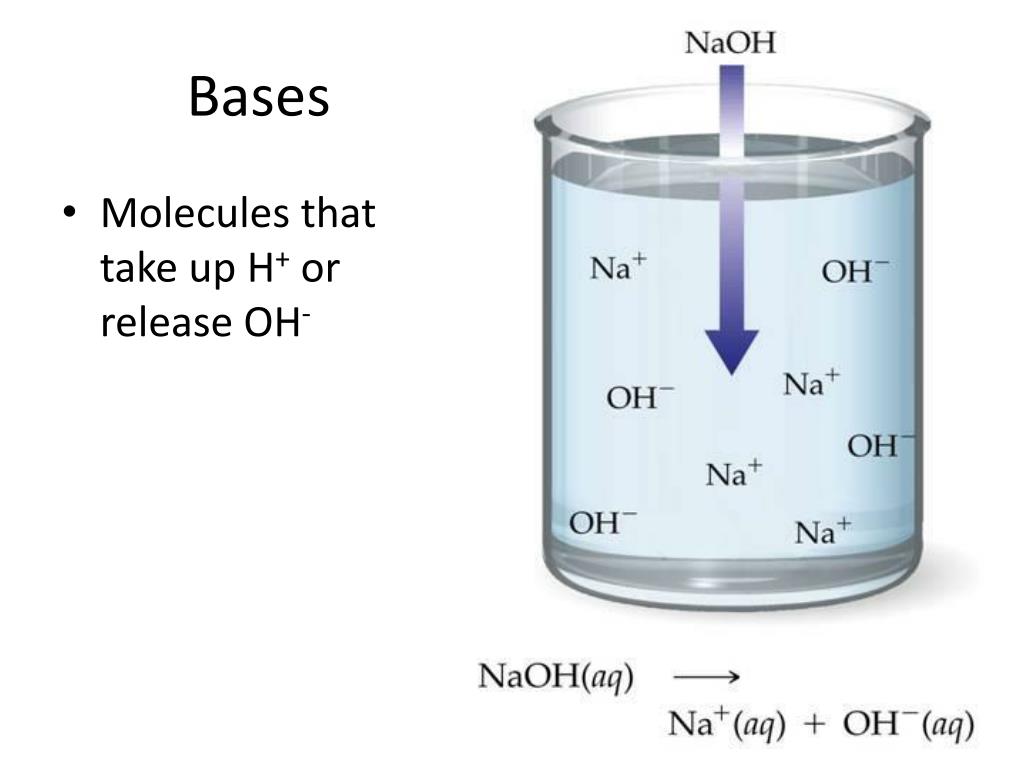
What Happens When A Base Reacts With Water . If hydrochloric acid (hcl) and the base sodium hydroxide (naoh) are combined, the product is h 2 o (water) and nacl (sodium chloride, a table salt). An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). A water molecule can act as an acid or a base even in a sample of pure water. The alkali metals react with water to produce a metal hydroxide and hydrogen. About 6 in every 100 million (6 in 10 8 ) water molecules undergo. They are also known to have a bitter taste as well as a slippery or soapy texture. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Bases are substances that taste bitter and change the colour of red litmus paper to blue. For example, sodium reacts with water: Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually.
from www.slideserve.com
They are also known to have a bitter taste as well as a slippery or soapy texture. Bases are substances that taste bitter and change the colour of red litmus paper to blue. A water molecule can act as an acid or a base even in a sample of pure water. Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. For example, sodium reacts with water: Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). About 6 in every 100 million (6 in 10 8 ) water molecules undergo. If hydrochloric acid (hcl) and the base sodium hydroxide (naoh) are combined, the product is h 2 o (water) and nacl (sodium chloride, a table salt). The alkali metals react with water to produce a metal hydroxide and hydrogen.
PPT Dissociation of water PowerPoint Presentation, free download ID
What Happens When A Base Reacts With Water If hydrochloric acid (hcl) and the base sodium hydroxide (naoh) are combined, the product is h 2 o (water) and nacl (sodium chloride, a table salt). An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). The alkali metals react with water to produce a metal hydroxide and hydrogen. A water molecule can act as an acid or a base even in a sample of pure water. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. If hydrochloric acid (hcl) and the base sodium hydroxide (naoh) are combined, the product is h 2 o (water) and nacl (sodium chloride, a table salt). They are also known to have a bitter taste as well as a slippery or soapy texture. Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. About 6 in every 100 million (6 in 10 8 ) water molecules undergo. For example, sodium reacts with water: Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. Bases are substances that taste bitter and change the colour of red litmus paper to blue.
From www.chemistrysteps.com
Reactions of Aldehydes and Ketones with Water Chemistry Steps What Happens When A Base Reacts With Water Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical. What Happens When A Base Reacts With Water.
From www.alamy.com
Acidbase reaction. chemical reaction neutralization the acid and base What Happens When A Base Reacts With Water The alkali metals react with water to produce a metal hydroxide and hydrogen. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). Conversely, strong bases react completely with water to. What Happens When A Base Reacts With Water.
From www.youtube.com
Equation for Sodium Carbonate Dissolving in Water (Na2CO3 + H2O) YouTube What Happens When A Base Reacts With Water Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical. What Happens When A Base Reacts With Water.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board What Happens When A Base Reacts With Water A water molecule can act as an acid or a base even in a sample of pure water. They are also known to have a bitter taste as well as a slippery or soapy texture. Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. Bases are substances. What Happens When A Base Reacts With Water.
From www.teachoo.com
How does a Base react in Water (aqueous solution)? Teachoo Chemistry What Happens When A Base Reacts With Water If hydrochloric acid (hcl) and the base sodium hydroxide (naoh) are combined, the product is h 2 o (water) and nacl (sodium chloride, a table salt). A water molecule can act as an acid or a base even in a sample of pure water. They are also known to have a bitter taste as well as a slippery or soapy. What Happens When A Base Reacts With Water.
From www.chegg.com
Solved Ammonia (acting as a base) reacts with water (acting What Happens When A Base Reacts With Water Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. Bases are substances that taste bitter and change the colour of red litmus paper to blue. They are also known to have a bitter taste as well as a slippery or soapy texture. Strong acids react. What Happens When A Base Reacts With Water.
From www.slideserve.com
PPT Ch 17. J.C. Rowe PowerPoint Presentation ID3099881 What Happens When A Base Reacts With Water Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. About 6 in every 100 million (6 in 10 8 ) water molecules undergo. Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form. What Happens When A Base Reacts With Water.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW What Happens When A Base Reacts With Water Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. A water molecule can act as an acid or a base even in a sample of pure water. Bases are substances that taste bitter and change the colour of red litmus paper to blue. Neutralization is. What Happens When A Base Reacts With Water.
From app.jove.com
Watera BronstedLowry Acid and Base; Autoionization of Water Concept What Happens When A Base Reacts With Water For example, sodium reacts with water: Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. Bases are substances that taste bitter and change the colour of red litmus paper to blue. An acid in a water solution tastes sour, changes the colour of blue litmus paper to. What Happens When A Base Reacts With Water.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo What Happens When A Base Reacts With Water Bases are substances that taste bitter and change the colour of red litmus paper to blue. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water. What Happens When A Base Reacts With Water.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases What Happens When A Base Reacts With Water A water molecule can act as an acid or a base even in a sample of pure water. Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate. What Happens When A Base Reacts With Water.
From www.slideserve.com
PPT Dissociation of water PowerPoint Presentation, free download ID What Happens When A Base Reacts With Water The alkali metals react with water to produce a metal hydroxide and hydrogen. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). Neutralization is a type of reaction in which. What Happens When A Base Reacts With Water.
From abhyasonline.in
Concept Explanation What Happens When A Base Reacts With Water They are also known to have a bitter taste as well as a slippery or soapy texture. About 6 in every 100 million (6 in 10 8 ) water molecules undergo. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Neutralization is a type of. What Happens When A Base Reacts With Water.
From saylordotorg.github.io
Water Both an Acid and a Base What Happens When A Base Reacts With Water A water molecule can act as an acid or a base even in a sample of pure water. For example, sodium reacts with water: They are also known to have a bitter taste as well as a slippery or soapy texture. Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with. What Happens When A Base Reacts With Water.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses What Happens When A Base Reacts With Water The alkali metals react with water to produce a metal hydroxide and hydrogen. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. Bases. What Happens When A Base Reacts With Water.
From www.youtube.com
Chemistry Acid or base in water solution Acids, bases and salts What Happens When A Base Reacts With Water For example, sodium reacts with water: The alkali metals react with water to produce a metal hydroxide and hydrogen. Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with. What Happens When A Base Reacts With Water.
From www.teachoo.com
Reaction of Metals and Nonmetals With Base Teachoo Concepts What Happens When A Base Reacts With Water About 6 in every 100 million (6 in 10 8 ) water molecules undergo. If hydrochloric acid (hcl) and the base sodium hydroxide (naoh) are combined, the product is h 2 o (water) and nacl (sodium chloride, a table salt). Bases are substances that taste bitter and change the colour of red litmus paper to blue. They are also known. What Happens When A Base Reacts With Water.
From www.youtube.com
Acid base reactions with water YouTube What Happens When A Base Reacts With Water Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. Bases are substances that taste bitter and change the colour of red litmus paper to blue. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate. What Happens When A Base Reacts With Water.
From www.teachoo.com
Combination Reaction Definition with 4 Examples Class 10 Chemistry What Happens When A Base Reacts With Water A water molecule can act as an acid or a base even in a sample of pure water. Bases are substances that taste bitter and change the colour of red litmus paper to blue. Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. If hydrochloric acid (hcl). What Happens When A Base Reacts With Water.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089759 What Happens When A Base Reacts With Water A water molecule can act as an acid or a base even in a sample of pure water. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). Bases are substances. What Happens When A Base Reacts With Water.
From study.com
The AcidBase Properties of Water Video & Lesson Transcript What Happens When A Base Reacts With Water An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). About 6 in every 100 million (6 in 10 8 ) water molecules undergo. If hydrochloric acid (hcl) and the base. What Happens When A Base Reacts With Water.
From www.slideserve.com
PPT The Reaction of Calcium with Water PowerPoint Presentation, free What Happens When A Base Reacts With Water An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). The alkali metals react with water to produce a metal hydroxide and hydrogen. Bases are substances that taste bitter and change. What Happens When A Base Reacts With Water.
From zabir.ru
Water reaction What Happens When A Base Reacts With Water They are also known to have a bitter taste as well as a slippery or soapy texture. Bases are substances that taste bitter and change the colour of red litmus paper to blue. A water molecule can act as an acid or a base even in a sample of pure water. About 6 in every 100 million (6 in 10. What Happens When A Base Reacts With Water.
From www.sliderbase.com
Buffers Presentation Chemistry What Happens When A Base Reacts With Water For example, sodium reacts with water: A water molecule can act as an acid or a base even in a sample of pure water. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Neutralization is a type of reaction in which acids react with bases. What Happens When A Base Reacts With Water.
From blueagents.weebly.com
Last Reaction And Water Bases blueagents What Happens When A Base Reacts With Water Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. They are also known to have a bitter taste as well as a slippery. What Happens When A Base Reacts With Water.
From www.animalia-life.club
Water Cohesion Diagram What Happens When A Base Reacts With Water Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. Bases are substances that taste bitter and change the colour of red litmus paper to blue. A water molecule can act as an acid or a base even in a sample of pure water. About 6 in every. What Happens When A Base Reacts With Water.
From byjus.com
does aluminium react with water What Happens When A Base Reacts With Water A water molecule can act as an acid or a base even in a sample of pure water. The alkali metals react with water to produce a metal hydroxide and hydrogen. Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. For example, sodium reacts with. What Happens When A Base Reacts With Water.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs What Happens When A Base Reacts With Water They are also known to have a bitter taste as well as a slippery or soapy texture. Bases are substances that taste bitter and change the colour of red litmus paper to blue. Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Neutralization is a. What Happens When A Base Reacts With Water.
From sciencenotes.org
Sodium in Water Chemistry Demonstration What Happens When A Base Reacts With Water A water molecule can act as an acid or a base even in a sample of pure water. They are also known to have a bitter taste as well as a slippery or soapy texture. Bases are substances that taste bitter and change the colour of red litmus paper to blue. Neutralization is a type of reaction in which acids. What Happens When A Base Reacts With Water.
From study.com
How to Identify Acid and Base Reactions in Water Chemistry What Happens When A Base Reacts With Water About 6 in every 100 million (6 in 10 8 ) water molecules undergo. They are also known to have a bitter taste as well as a slippery or soapy texture. Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. For example, sodium reacts with. What Happens When A Base Reacts With Water.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo What Happens When A Base Reacts With Water If hydrochloric acid (hcl) and the base sodium hydroxide (naoh) are combined, the product is h 2 o (water) and nacl (sodium chloride, a table salt). Neutralization is a type of reaction in which acids react with bases to form a salt solution (water and a salt), usually. Conversely, strong bases react completely with water to produce the hydroxide ion,. What Happens When A Base Reacts With Water.
From www.dreamstime.com
Acid base reaction stock vector. Illustration of chemistry 200906334 What Happens When A Base Reacts With Water If hydrochloric acid (hcl) and the base sodium hydroxide (naoh) are combined, the product is h 2 o (water) and nacl (sodium chloride, a table salt). They are also known to have a bitter taste as well as a slippery or soapy texture. Bases are substances that taste bitter and change the colour of red litmus paper to blue. A. What Happens When A Base Reacts With Water.
From www.numerade.com
Metal oxides can react with water to form bases. What Happens When A Base Reacts With Water About 6 in every 100 million (6 in 10 8 ) water molecules undergo. Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g.,. What Happens When A Base Reacts With Water.
From www.youtube.com
What happens to and Acid or Base in Water Solution?Acid Bases and What Happens When A Base Reacts With Water A water molecule can act as an acid or a base even in a sample of pure water. An acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form salts, and promotes certain chemical reactions (acid catalysis). Neutralization is a. What Happens When A Base Reacts With Water.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo What Happens When A Base Reacts With Water Strong acids react completely with water to produce h 3 o + (aq) (the hydronium ion), whereas weak acids dissociate only partially in water. For example, sodium reacts with water: The alkali metals react with water to produce a metal hydroxide and hydrogen. Neutralization is a type of reaction in which acids react with bases to form a salt solution. What Happens When A Base Reacts With Water.