Bohr Model Limitations . Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Limitations of bohr atomic model theory. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. The following are his key contributions to our. It violates the heisenberg uncertainty principle. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. The bohr atomic model theory. Limitations of bohr’s atomic model. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. Find out the postulates, merits and limitations of this model, and how it differs from rutherford’s model. The following are the basic limitations of bohr’s model of the hydrogen atom. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. It does not comply with the.
from bilag.xxl.no
It does not comply with the. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. The bohr atomic model theory. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. It violates the heisenberg uncertainty principle. Limitations of bohr’s atomic model. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. The following are his key contributions to our. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory.
How To Draw The Bohr Model
Bohr Model Limitations It violates the heisenberg uncertainty principle. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Limitations of bohr’s atomic model. It does not comply with the. Limitations of bohr atomic model theory. It violates the heisenberg uncertainty principle. The bohr atomic model theory. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. The following are the basic limitations of bohr’s model of the hydrogen atom. Find out the postulates, merits and limitations of this model, and how it differs from rutherford’s model. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. The following are his key contributions to our.
From www.studypool.com
SOLUTION Bohr Model and limitations Studypool Bohr Model Limitations Limitations of bohr’s atomic model. The following are the basic limitations of bohr’s model of the hydrogen atom. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. The following are. Bohr Model Limitations.
From pdfprof.com
niels bohr Bohr Model Limitations Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. It violates the heisenberg uncertainty principle. Limitations of. Bohr Model Limitations.
From scienceready.com.au
Bohr's Atomic Model & Limitations HSC Physics Science Ready Bohr Model Limitations Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. It violates the heisenberg uncertainty principle. The following are his key contributions to our. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. In this article, we will. Bohr Model Limitations.
From quarkscience.com
Bohr's Atomic Model Postulates Diagram Limitations Bohr Model Limitations The bohr atomic model theory. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. The following are his key contributions to our. It does not comply with the. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. Bohr’s model of the. Bohr Model Limitations.
From chemistnotes.com
Bohr's Atomic Model, Postulates, Merits and Limitations Chemistry Notes Bohr Model Limitations It does not comply with the. The bohr atomic model theory. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. Limitations of bohr’s atomic model. It violates. Bohr Model Limitations.
From slideplayer.com
The Spectrum and the Model of the Atom Part I ppt Bohr Model Limitations The following are the basic limitations of bohr’s model of the hydrogen atom. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. In this article, we will explore the strengths. Bohr Model Limitations.
From worksheetcampusmantra.z13.web.core.windows.net
Bohr Atomic Model Explained Bohr Model Limitations Limitations of bohr’s atomic model. The following are the basic limitations of bohr’s model of the hydrogen atom. Find out the postulates, merits and limitations of this model, and how it differs from rutherford’s model. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. The following are his key contributions to our. Bohr’s model. Bohr Model Limitations.
From www.youtube.com
Atomic Spectra (Part 2 of 2) The Bohr Model and its Limitations YouTube Bohr Model Limitations The following are the basic limitations of bohr’s model of the hydrogen atom. It does not comply with the. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions. Bohr Model Limitations.
From www.youtube.com
Limitations of Bohr model lecture 17 YouTube Bohr Model Limitations It violates the heisenberg uncertainty principle. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. The following are his key contributions to our.. Bohr Model Limitations.
From www.youtube.com
Limitations of Bohr Model, Chapter 12, Atoms, Class 12 Physics YouTube Bohr Model Limitations Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. Limitations of bohr’s atomic model. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. Bohr’s. Bohr Model Limitations.
From www.toppr.com
Bohr's model of atom explains Chemistry Questions Bohr Model Limitations It does not comply with the. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen. Bohr Model Limitations.
From www.slideserve.com
PPT Atomic Structure and Bonding in Materials PowerPoint Presentation Bohr Model Limitations It does not comply with the. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. Limitations of bohr’s atomic model. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. The following are his key contributions to our. Limitations of bohr atomic model theory.. Bohr Model Limitations.
From www.youtube.com
Bohr Atomic Model Features and Limitations YouTube Bohr Model Limitations The following are the basic limitations of bohr’s model of the hydrogen atom. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. It. Bohr Model Limitations.
From slideplayer.com
The Bohr Model of the Atom ppt download Bohr Model Limitations Limitations of bohr’s atomic model. Find out the postulates, merits and limitations of this model, and how it differs from rutherford’s model. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. It. Bohr Model Limitations.
From www.youtube.com
Limitations of Bohr's theory of hydrogen atom YouTube Bohr Model Limitations Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. The following are his key contributions to our. Limitations of bohr atomic model theory. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. It does not comply with the. Limitations of bohr’s atomic model. The following are. Bohr Model Limitations.
From www.slideshare.net
Atomic structure Bohr Model Limitations Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. The following are the basic limitations of bohr’s model of the hydrogen atom. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. In this article, we will explore. Bohr Model Limitations.
From askfilo.com
Limitations of Bohr's theory Alshough Bohr's theory was successful in exp.. Bohr Model Limitations The following are his key contributions to our. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first. Bohr Model Limitations.
From scienceready.com.au
M8S7 Bohr's and Rutherford's Atomic Models and their Limitations Bohr Model Limitations Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. The bohr atomic model theory. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. Limitations of bohr’s atomic model. Limitations of bohr atomic model theory. The following are the basic limitations of bohr’s model. Bohr Model Limitations.
From classnotes.org.in
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom Bohr Model Limitations Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. The following are the basic limitations of bohr’s model of the hydrogen atom. Limitations of bohr’s. Bohr Model Limitations.
From www.thoughtco.com
Bohr Model of the Atom Overview and Examples Bohr Model Limitations The following are the basic limitations of bohr’s model of the hydrogen atom. It does not comply with the. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. It violates the heisenberg uncertainty principle. Find out the postulates, merits and limitations of this model, and how it. Bohr Model Limitations.
From slideplayer.com
Chapter 6 Electronic Structure of Atoms ppt download Bohr Model Limitations Limitations of bohr’s atomic model. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission. Bohr Model Limitations.
From www.homeworkjoy.com
Niels Bohr Atomic Theory and Its Limitations Bohr Model Limitations Find out the postulates, merits and limitations of this model, and how it differs from rutherford’s model. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. It violates the heisenberg uncertainty principle. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Limitations of bohr atomic model. Bohr Model Limitations.
From www.youtube.com
7. 11C02.4 CV 4 Limitation of Bohr's Model YouTube Bohr Model Limitations In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. It violates the heisenberg uncertainty principle. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Find out the postulates, merits and limitations of this model, and how it differs from rutherford’s model.. Bohr Model Limitations.
From slideplayer.com
Electron Configurations ppt download Bohr Model Limitations Limitations of bohr atomic model theory. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. It violates the heisenberg uncertainty principle. The following are the basic limitations of bohr’s model of the hydrogen atom. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy,. Bohr Model Limitations.
From www.chemistrylearner.com
Bohr Model Definition, Features, and Limitations Bohr Model Limitations The following are his key contributions to our. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. Bohr’s model combines the classical mechanics of planetary motion with. Bohr Model Limitations.
From www.sliderbase.com
No energy can be transferred to Hg below 4.88 eV because not enough Bohr Model Limitations Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. Find out the postulates, merits and limitations of this model, and how it differs from rutherford’s model. In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. The following are the basic limitations of bohr’s. Bohr Model Limitations.
From www.expii.com
Bohr's Atomic Model — Overview & Importance Expii Bohr Model Limitations Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. The following are the basic limitations of bohr’s model of the hydrogen atom. It does not comply with the. Limitations of. Bohr Model Limitations.
From bilag.xxl.no
How To Draw The Bohr Model Bohr Model Limitations In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. Limitations of bohr’s atomic model. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. It does not comply with. Bohr Model Limitations.
From www.youtube.com
Bohr Atomic Model, Rydberg's Equation, Hydrogen Spectrum // HSC Physics Bohr Model Limitations Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Limitations of bohr atomic model theory. It does not comply with the. Learn about the bohr atomic model, which describes the. Bohr Model Limitations.
From www.doubtnut.com
Explain Bohr Sommerfeld model of an atom. What is the merit of this Bohr Model Limitations In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. It violates the. Bohr Model Limitations.
From www.tutoroot.com
What is Bohr's Model of an Atom? Diagram, Applications Bohr Model Limitations Limitations of bohr’s atomic model. It violates the heisenberg uncertainty principle. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. It does not comply with the. In this article, we will explore the advantages and disadvantages of. Bohr Model Limitations.
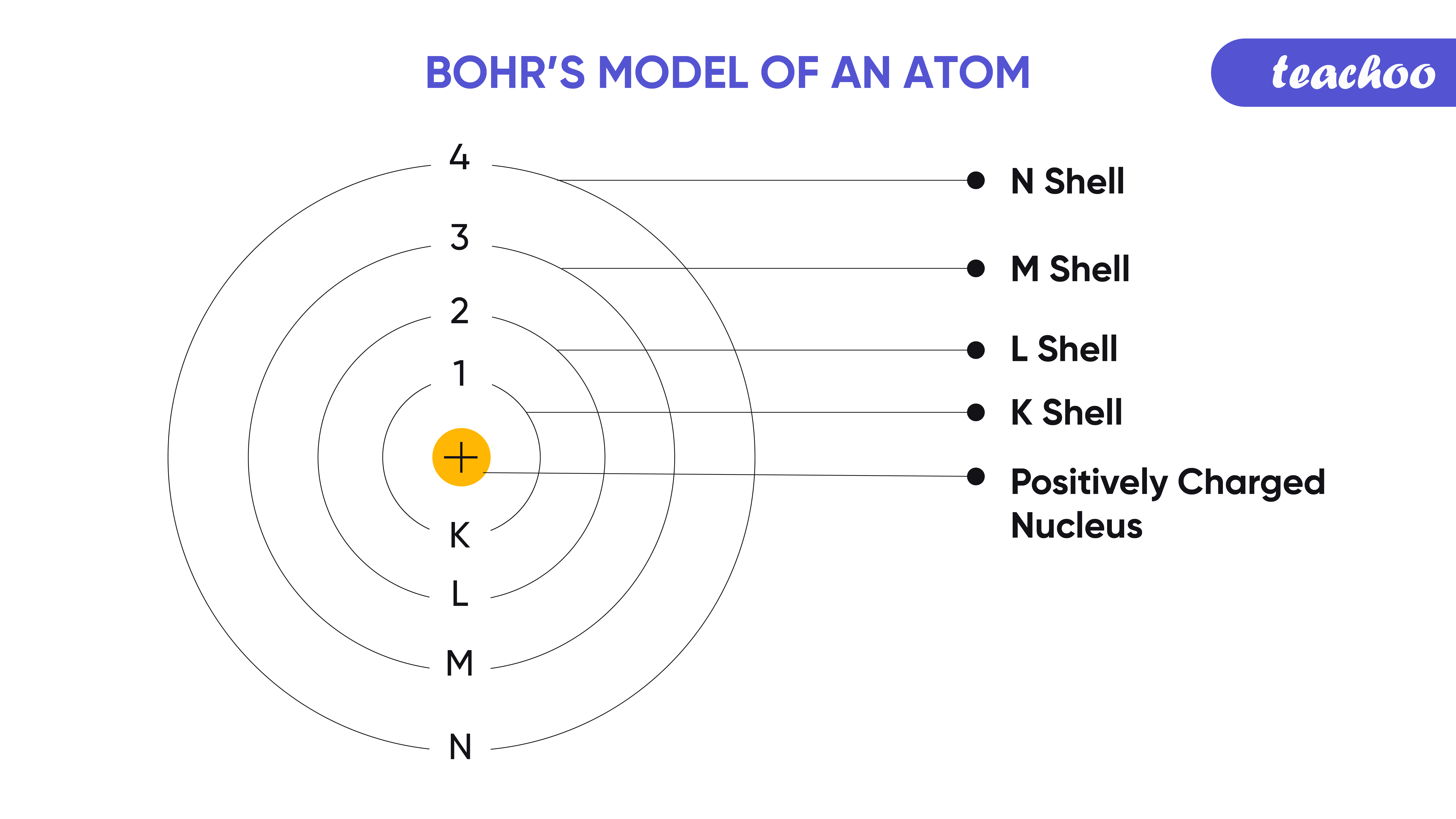
From www.teachoo.com
Bohr's Model of Atom Postulate and it's limitations Teachoo Bohr Model Limitations In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Find out the postulates, merits and limitations of this model, and how it differs. Bohr Model Limitations.
From chemistnotes.com
Bohr's Atomic Model, Postulates, Merits and Limitations Chemistry Notes Bohr Model Limitations The bohr atomic model theory. Bohr’s model combines the classical mechanics of planetary motion with the quantum concept of photons. Limitations of bohr’s atomic model. Learn about the bohr atomic model, which describes the structure of hydrogen atoms based on quantum theory. It violates the heisenberg uncertainty principle. Find out the postulates, merits and limitations of this model, and how. Bohr Model Limitations.
From www.worksheetsplanet.com
Bohr's Atomic Model Bohr Model Limitations In this article, we will explore the strengths and limitations of the bohr model, highlighting its contributions to atomic theory. Bohr’s model of the hydrogen atom, proposed by niels bohr in 1913, was the first quantum model that correctly explained the hydrogen emission spectrum. Find out the postulates, merits and limitations of this model, and how it differs from rutherford’s. Bohr Model Limitations.
From www.youtube.com
Defects of Bohr's Atomic Model Bohrs Atomic Theory Drawbacks Bohr Model Limitations In this article, we will explore the advantages and disadvantages of bohr’s atomic model, delve into calculations of radius, energy, velocity, and. Bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. The following are his key contributions to our. The bohr atomic model theory. Limitations of bohr’s atomic model. Limitations of bohr atomic model. Bohr Model Limitations.