What Is Fluorescence Example . The spin of the electron is still paired with the ground state electron, unlike phosphorescence. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when the atoms return to normal, in just a few nanoseconds, they give out the energy that excited them as tiny particles of light called photons. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). The simple kind of fluorescence is by dilute atomic vapors. Fluorescent materials are those that can exhibit this characteristic. Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a singlet excited state. A fluorescence example would be if a 3s. Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states. As the excited molecule returns to ground.
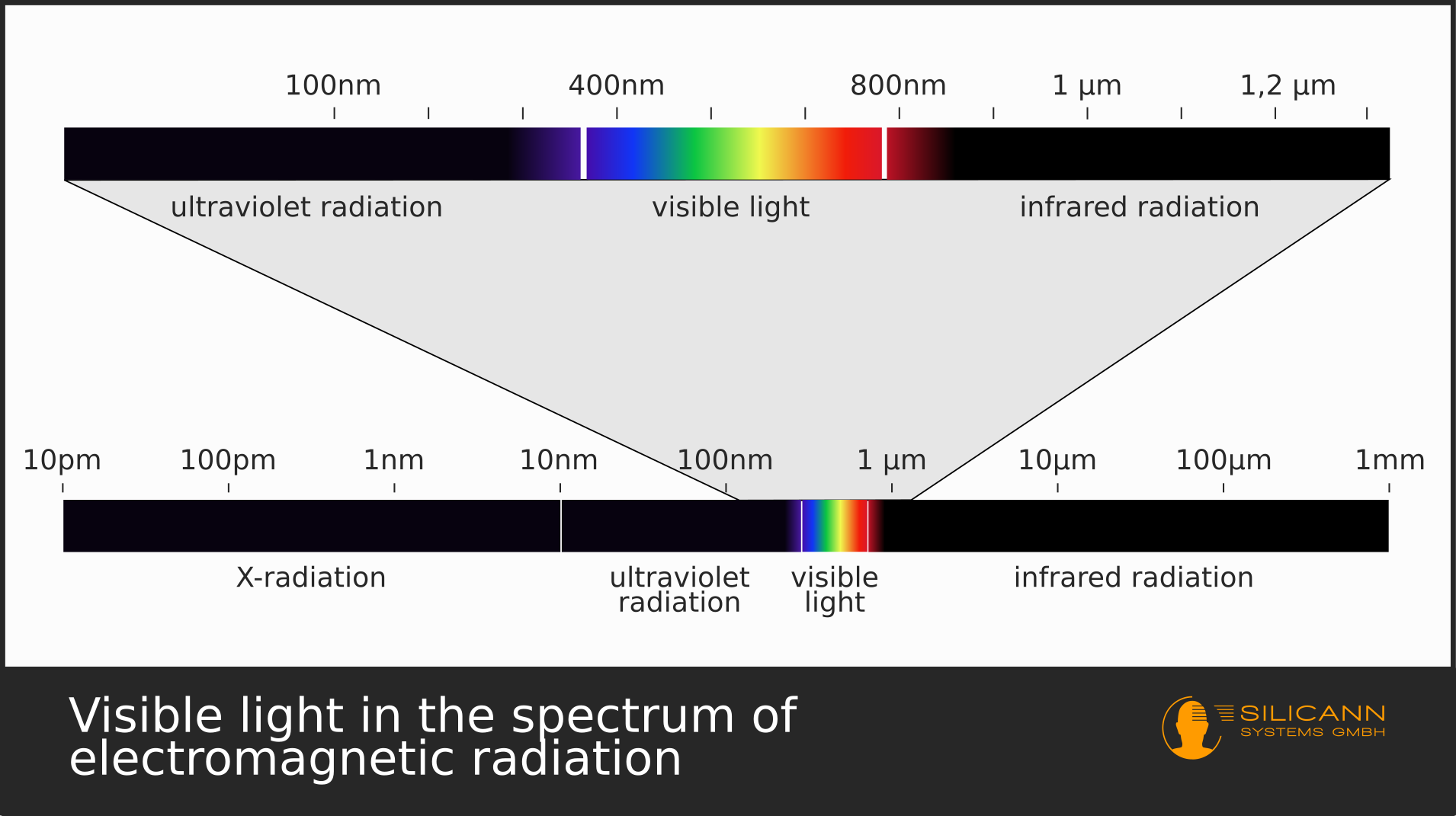
from www.en.silicann.com
Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when the atoms return to normal, in just a few nanoseconds, they give out the energy that excited them as tiny particles of light called photons. Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. The spin of the electron is still paired with the ground state electron, unlike phosphorescence. Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a singlet excited state. Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states. A fluorescence example would be if a 3s. The simple kind of fluorescence is by dilute atomic vapors. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. As the excited molecule returns to ground.
What is Fluorescence?
What Is Fluorescence Example As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states. Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. Fluorescent materials are those that can exhibit this characteristic. Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a singlet excited state. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when the atoms return to normal, in just a few nanoseconds, they give out the energy that excited them as tiny particles of light called photons. As the excited molecule returns to ground. The spin of the electron is still paired with the ground state electron, unlike phosphorescence. A fluorescence example would be if a 3s. The simple kind of fluorescence is by dilute atomic vapors.
From www.researchgate.net
Basics of Fluorescence and FRET. ( a ) Visible light spectrum What Is Fluorescence Example As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescence can occur in gaseous, liquid, and solid chemical systems. A fluorescence example would be if a 3s. The spin of the electron is still paired with the ground state electron, unlike phosphorescence. As the excited molecule returns to ground. Fluorescence, a type. What Is Fluorescence Example.
From www.thoughtco.com
Fluorescence Versus Phosphorescence What Is Fluorescence Example Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescent materials are those that can exhibit this characteristic. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when the atoms return to normal, in just a few nanoseconds, they give out the energy that excited them as tiny particles of light called photons.. What Is Fluorescence Example.
From www.slideserve.com
PPT Methods Fluorescence PowerPoint Presentation, free download ID What Is Fluorescence Example The simple kind of fluorescence is by dilute atomic vapors. Fluorescent materials are those that can exhibit this characteristic. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a singlet excited state. The spin of the electron. What Is Fluorescence Example.
From www.studio74.uk
Fluorescence explained What Is Fluorescence Example The simple kind of fluorescence is by dilute atomic vapors. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states. Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when. What Is Fluorescence Example.
From www.youtube.com
What is Fluorescence? Detailed Explanation. Amazing Glowing liquid What Is Fluorescence Example Fluorescence can occur in gaseous, liquid, and solid chemical systems. The simple kind of fluorescence is by dilute atomic vapors. Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a singlet excited state. The spin of the electron is still paired with the ground state electron, unlike phosphorescence. A fluorescence example would be if. What Is Fluorescence Example.
From www.slideshare.net
Fluorescence Microscopy What Is Fluorescence Example Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states. The simple kind of fluorescence is by dilute atomic vapors. As the excited molecule returns to ground. Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical. What Is Fluorescence Example.
From chemistnotes.com
Factors affecting fluorescence Principle, types Chemistry Notes What Is Fluorescence Example A fluorescence example would be if a 3s. The spin of the electron is still paired with the ground state electron, unlike phosphorescence. As the excited molecule returns to ground. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. The simple kind of fluorescence is by dilute atomic vapors. Fluorescence can occur. What Is Fluorescence Example.
From www.youtube.com
Fluorescence spectroscopy Difference between Fluorescence and What Is Fluorescence Example The simple kind of fluorescence is by dilute atomic vapors. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when the atoms return to normal, in just a few nanoseconds, they give out the energy that excited. What Is Fluorescence Example.
From www.slideserve.com
PPT Methods Fluorescence PowerPoint Presentation, free download ID What Is Fluorescence Example Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). Fluorescent materials are those that can exhibit this characteristic. Fluorescence can occur in. What Is Fluorescence Example.
From sciencenotes.org
Fluorescence Definition and Examples What Is Fluorescence Example Fluorescent materials are those that can exhibit this characteristic. The spin of the electron is still paired with the ground state electron, unlike phosphorescence. Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds).. What Is Fluorescence Example.
From www.tecan.com
How to develop an optimal fluorescence assay The Blog Tecan What Is Fluorescence Example The spin of the electron is still paired with the ground state electron, unlike phosphorescence. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). Fluorescent materials are those that can exhibit this characteristic. A fluorescence example would be if a 3s. Fluorescent materials. What Is Fluorescence Example.
From www.youtube.com
How Fluorescence Works The Science YouTube What Is Fluorescence Example As the excited molecule returns to ground. The simple kind of fluorescence is by dilute atomic vapors. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. The spin of the electron is still paired with the ground state electron, unlike phosphorescence. Fluorescence, a type of luminescence, occurs in gas, liquid or solid. What Is Fluorescence Example.
From www.slideserve.com
PPT What is Fluorescence? PowerPoint Presentation, free download ID What Is Fluorescence Example A fluorescence example would be if a 3s. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescent materials are those that can exhibit this characteristic. The simple kind of fluorescence is by dilute atomic vapors. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when. What Is Fluorescence Example.
From chemistnotes.com
Atomic Fluorescence Spectroscopy Principle, Instrumentation, and 7 What Is Fluorescence Example As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light. What Is Fluorescence Example.
From www.slideserve.com
PPT Fluorescence a type of light emission PowerPoint Presentation What Is Fluorescence Example Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. The simple kind of fluorescence is by dilute atomic vapors. As the excited molecule returns to ground. Fluorescence can occur in gaseous, liquid, and solid chemical systems. A fluorescence example would be if a 3s. Fluorescence is brought about by absorption of photons in the singlet ground. What Is Fluorescence Example.
From bitesizebio.com
Fluorescence Microscopy An Easy Guide for Biologists What Is Fluorescence Example Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). As the excited molecule returns to ground. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states. A fluorescence example would be if. What Is Fluorescence Example.
From rsscience.com
Fluorescence Microscope Rs' Science What Is Fluorescence Example The spin of the electron is still paired with the ground state electron, unlike phosphorescence. As the excited molecule returns to ground. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms. What Is Fluorescence Example.
From agora.cs.wcu.edu
Fluorescence/Phosphorescence/Chemiluminescences Lectures What Is Fluorescence Example Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescent materials are those that can exhibit this characteristic. Fluorescence is a member of the ubiquitous luminescence. What Is Fluorescence Example.
From www.slideserve.com
PPT Principle of fluorescence PowerPoint Presentation, free download What Is Fluorescence Example Fluorescence can occur in gaseous, liquid, and solid chemical systems. The spin of the electron is still paired with the ground state electron, unlike phosphorescence. Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. As the excited molecule returns to ground. The simple kind of fluorescence is by dilute atomic vapors. As mentioned earlier, fluorescence is. What Is Fluorescence Example.
From www.slideserve.com
PPT DNA Photonics PowerPoint Presentation ID1922944 What Is Fluorescence Example Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a singlet excited state. Fluorescent materials are those that can exhibit this characteristic. Fluorescence can occur in gaseous, liquid, and solid chemical systems. A fluorescence example would be if a 3s. Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. Fluorescence. What Is Fluorescence Example.
From www.compadre.org
Fluorescence examples Nexus Wiki What Is Fluorescence Example As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. The spin of the electron is still paired with the ground state electron, unlike phosphorescence. Fluorescent materials are those that can exhibit this characteristic. As the excited molecule returns to ground. Fluorescent materials produce light instantly, when the atoms inside them absorb energy. What Is Fluorescence Example.
From firedivegear.com
Science of Fluorescence and Fluorescent Light Photography What Is Fluorescence Example Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). The spin of the electron is still paired with the ground state electron, unlike phosphorescence. Fluorescence is a member of the. What Is Fluorescence Example.
From www.horiba.com
Fluorescence Microscopy HORIBA What Is Fluorescence Example Fluorescence can occur in gaseous, liquid, and solid chemical systems. A fluorescence example would be if a 3s. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. As the excited molecule returns to ground. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from. What Is Fluorescence Example.
From www.scribd.com
FLUORESCENCE AND PHOSPHORESCENCE Fluorescence Chemistry What Is Fluorescence Example The simple kind of fluorescence is by dilute atomic vapors. As the excited molecule returns to ground. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescent materials are those that can exhibit this characteristic. A fluorescence example would be if a 3s. Fluorescence, a type of luminescence, occurs in gas, liquid. What Is Fluorescence Example.
From www.slideserve.com
PPT What is Fluorescence? PowerPoint Presentation, free download ID What Is Fluorescence Example Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when the atoms return to normal, in just a few nanoseconds, they give out the energy that excited them as tiny particles of light called photons. As mentioned earlier, fluorescence is a. What Is Fluorescence Example.
From www.britannica.com
Fluorescent lamp Definition, Types, & Facts Britannica What Is Fluorescence Example As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. The simple kind of fluorescence is by dilute atomic vapors. Fluorescence can occur in gaseous, liquid, and solid chemical systems. As the excited molecule returns to ground. Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a. What Is Fluorescence Example.
From goldbio.com
Fluorescence microscopy A Basic Introduction GoldBio What Is Fluorescence Example Fluorescent materials are those that can exhibit this characteristic. As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). Fluorescent materials produce light instantly, when the atoms. What Is Fluorescence Example.
From www.aureusboutique.com
What is fluorescence in diamonds? Aureus Boutique What Is Fluorescence Example The simple kind of fluorescence is by dilute atomic vapors. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states. Fluorescent materials are those that can exhibit this characteristic. A fluorescence example would be if a 3s. Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescence, a. What Is Fluorescence Example.
From www.slideserve.com
PPT Fluorescent Microscopy in Diagnostic Microbiology PowerPoint What Is Fluorescence Example Fluorescence can occur in gaseous, liquid, and solid chemical systems. As the excited molecule returns to ground. Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). The spin of the electron is still paired with the ground state electron, unlike phosphorescence. The simple. What Is Fluorescence Example.
From www.slideserve.com
PPT DNA Photonics PowerPoint Presentation ID1922944 What Is Fluorescence Example As mentioned earlier, fluorescence is a temporary physical phenomenon where the atoms of a substance get energized. A fluorescence example would be if a 3s. As the excited molecule returns to ground. Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from. What Is Fluorescence Example.
From www.youtube.com
How Does Fluorescence Work? YouTube What Is Fluorescence Example Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. The simple kind of fluorescence is by dilute atomic vapors. Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states. Fluorescence is brought about by absorption. What Is Fluorescence Example.
From www.majordifferences.com
7 Differences between Fluorescence and Phosphorescence What Is Fluorescence Example As the excited molecule returns to ground. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when the atoms return to normal, in just a few nanoseconds, they give out the energy that excited them as tiny particles of light called photons. The spin of the electron is still paired with the ground state. What Is Fluorescence Example.
From www.en.silicann.com
What is Fluorescence? What Is Fluorescence Example Fluorescence, emission of electromagnetic radiation, usually visible light, caused by excitation of atoms in a material, which then reemit almost immediately (within about 10 −8 seconds). Fluorescence is brought about by absorption of photons in the singlet ground state promoted to a singlet excited state. Fluorescence, a type of luminescence, occurs in gas, liquid or solid chemical systems. The spin. What Is Fluorescence Example.
From www.en.silicann.com
What is Fluorescence? What Is Fluorescence Example The simple kind of fluorescence is by dilute atomic vapors. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when the atoms return to normal, in just a few nanoseconds, they give out the energy that excited them as tiny particles of light called photons. Fluorescence can occur in gaseous, liquid, and solid chemical. What Is Fluorescence Example.
From www.thoughtco.com
What Is Fluorescence? What Is Fluorescence Example Fluorescence can occur in gaseous, liquid, and solid chemical systems. Fluorescent materials produce light instantly, when the atoms inside them absorb energy and become excited. when the atoms return to normal, in just a few nanoseconds, they give out the energy that excited them as tiny particles of light called photons. A fluorescence example would be if a 3s. As. What Is Fluorescence Example.