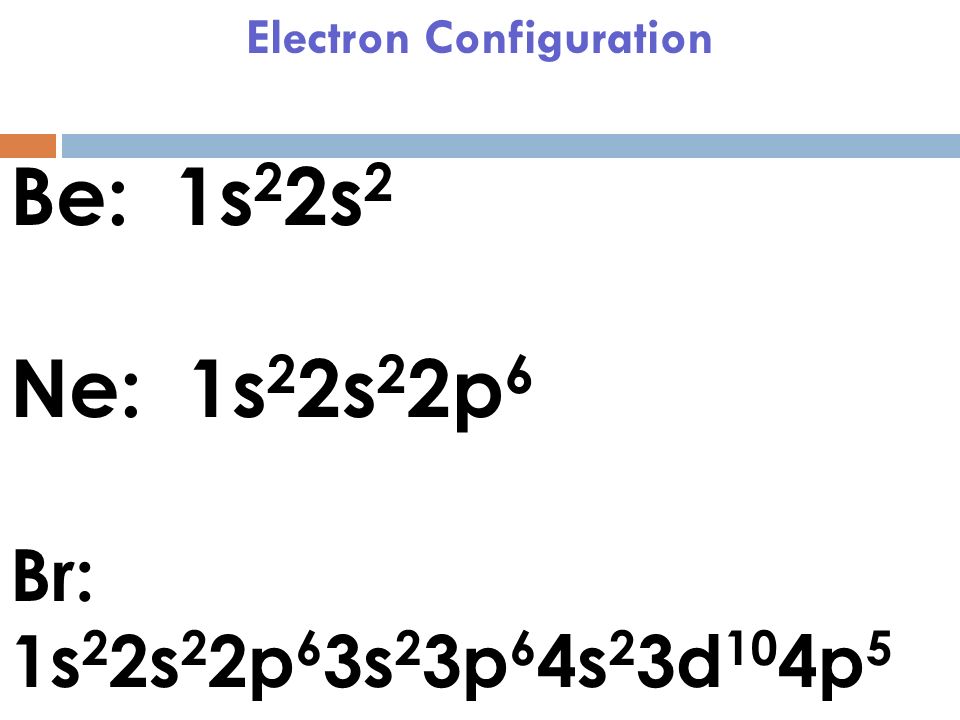
What Is The Ground State Electron Configuration Of Bromine (Br) . You can also see the image given below; From the electrons in an atom, to the differing. This can be shortened to [ar]4s23d104p5. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Electron configuration chart of all elements is mentioned in the table below. First, find electrons of bromine atom. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Use a chart such as the one below. The atomic number of bromine represents the total number of electrons of bromine. Since the atomic number of bromine is 35, the total electrons of bromine are 35. The shorthand electron configuration (or noble gas configuration) as well as. Second, make a table of subshell and its maximum electrons. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5.
from periodictable.me
This can be shortened to [ar]4s23d104p5. Electron configuration chart of all elements is mentioned in the table below. You can also see the image given below; 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. From the electrons in an atom, to the differing. The atomic number of bromine represents the total number of electrons of bromine. Second, make a table of subshell and its maximum electrons. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. First, find electrons of bromine atom.
How Do We Find The Electron Configuration For Bromine Dynamic
What Is The Ground State Electron Configuration Of Bromine (Br) The shorthand electron configuration (or noble gas configuration) as well as. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Since the atomic number of bromine is 35, the total electrons of bromine are 35. The atomic number of bromine represents the total number of electrons of bromine. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. First, find electrons of bromine atom. You can also see the image given below; The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration chart of all elements is mentioned in the table below. This can be shortened to [ar]4s23d104p5. From the electrons in an atom, to the differing. The shorthand electron configuration (or noble gas configuration) as well as. Second, make a table of subshell and its maximum electrons. Use a chart such as the one below.
From www.animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Electron configuration chart of all elements is mentioned in the table below. You can also see the image given below; Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the differing. This. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry What Is The Ground State Electron Configuration Of Bromine (Br) The atomic number of bromine represents the total number of electrons of bromine. This can be shortened to [ar]4s23d104p5. Electron configuration chart of all elements is mentioned in the table below. From the electrons in an atom, to the differing. You can also see the image given below; Use a chart such as the one below. First, find electrons of. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. You can also see the image given below; From the electrons in an atom, to the differing. This can be shortened to [ar]4s23d104p5. First, find electrons of bromine atom. Electron. What Is The Ground State Electron Configuration Of Bromine (Br).
From brandonkss.github.io
Ground State Electron Configuration Chart What Is The Ground State Electron Configuration Of Bromine (Br) Electron configuration chart of all elements is mentioned in the table below. First, find electrons of bromine atom. Use a chart such as the one below. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Since the atomic number of bromine is 35, the total electrons of bromine are 35. This can be. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.youtube.com
Electron Configuration of Bromine, Br YouTube What Is The Ground State Electron Configuration Of Bromine (Br) Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. First, find electrons of bromine atom. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. The shorthand electron configuration (or noble gas configuration) as well as. Since the atomic number of bromine is 35, the. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube What Is The Ground State Electron Configuration Of Bromine (Br) The shorthand electron configuration (or noble gas configuration) as well as. First, find electrons of bromine atom. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The atomic number of bromine represents the total number of electrons of bromine. Second, make a table of subshell and its maximum electrons. You can also see. What Is The Ground State Electron Configuration Of Bromine (Br).
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic What Is The Ground State Electron Configuration Of Bromine (Br) Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. You can also see the image given below; First, find electrons of bromine atom. Since the atomic number of bromine is 35, the total electrons of bromine are 35. Second, make a table of subshell and its maximum electrons. Ground. What Is The Ground State Electron Configuration Of Bromine (Br).
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram What Is The Ground State Electron Configuration Of Bromine (Br) Second, make a table of subshell and its maximum electrons. Since the atomic number of bromine is 35, the total electrons of bromine are 35. Use a chart such as the one below. The shorthand electron configuration (or noble gas configuration) as well as. The atomic number of bromine represents the total number of electrons of bromine. From the electrons. What Is The Ground State Electron Configuration Of Bromine (Br).
From animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Use a chart such as the one below. From the electrons in an atom, to the differing. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The atomic number of bromine represents the total number of electrons of bromine. Electron configuration chart of all elements is. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms What Is The Ground State Electron Configuration Of Bromine (Br) Use a chart such as the one below. The atomic number of bromine represents the total number of electrons of bromine. From the electrons in an atom, to the differing. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart. What Is The Ground State Electron Configuration Of Bromine (Br).
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram What Is The Ground State Electron Configuration Of Bromine (Br) The shorthand electron configuration (or noble gas configuration) as well as. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5.. What Is The Ground State Electron Configuration Of Bromine (Br).
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) What Is The Ground State Electron Configuration Of Bromine (Br) Use a chart such as the one below. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. You can also see the image given below; From the electrons in an atom, to the differing. Second, make a table of subshell and its maximum electrons. Electron configuration chart of all elements is mentioned in the table below. This can be shortened to [ar]4s23d104p5.. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) Second, make a table of subshell and its maximum electrons. Since the atomic number of bromine is 35, the total electrons of bromine are 35. You can also see the image given below; Use a chart such as the one below. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end. What Is The Ground State Electron Configuration Of Bromine (Br).
From ar.inspiredpencil.com
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. You can also see the image given below; Since the atomic number of bromine is 35, the total electrons of bromine are 35. The atomic number of bromine represents the total number of electrons of bromine. Bromine. What Is The Ground State Electron Configuration Of Bromine (Br).
From answers.yahoo.com
What is the groundstate electron configuration of bromine? Yahoo Answers What Is The Ground State Electron Configuration Of Bromine (Br) The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. You can also see the image given below; 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Since the atomic number of bromine is 35, the total electrons of bromine are 35. From the electrons in an atom, to the differing. The atomic number of bromine. What Is The Ground State Electron Configuration Of Bromine (Br).
From general.chemistrysteps.com
Electron Configurations Chemistry Steps What Is The Ground State Electron Configuration Of Bromine (Br) The shorthand electron configuration (or noble gas configuration) as well as. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Second, make a table of subshell and its maximum electrons. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. You can also see the. What Is The Ground State Electron Configuration Of Bromine (Br).
From kizakingdom.weebly.com
Ground state electron configuration kizakingdom What Is The Ground State Electron Configuration Of Bromine (Br) Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Second, make a table of subshell and its maximum electrons. First, find electrons of bromine atom. Electron configuration chart of all elements is mentioned in the table below. From the electrons in an atom, to the differing. The electron configuration. What Is The Ground State Electron Configuration Of Bromine (Br).
From geteducationskills.com
Ground State Electron Configuration An Overview Get Education What Is The Ground State Electron Configuration Of Bromine (Br) This can be shortened to [ar]4s23d104p5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Use a chart such as the one below. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine is in the 4th energy level, p block,. What Is The Ground State Electron Configuration Of Bromine (Br).
From ar.inspiredpencil.com
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) First, find electrons of bromine atom. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. This can be shortened to [ar]4s23d104p5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Use a chart such as the one. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica What Is The Ground State Electron Configuration Of Bromine (Br) First, find electrons of bromine atom. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. You can also see the image given below; From the electrons in an atom, to the differing. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Since the atomic number of bromine is 35, the total. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements What Is The Ground State Electron Configuration Of Bromine (Br) First, find electrons of bromine atom. Electron configuration chart of all elements is mentioned in the table below. This can be shortened to [ar]4s23d104p5. Second, make a table of subshell and its maximum electrons. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Use a chart such as the one below. The shorthand electron configuration. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) Use a chart such as the one below. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. First, find electrons of bromine atom. Second, make a table of subshell and its maximum electrons. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) First, find electrons of bromine atom. From the electrons in an atom, to the differing. Electron configuration chart of all elements is mentioned in the table below. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Use a chart such as the one below. Second, make a table of subshell and its maximum electrons. Bromine. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic What Is The Ground State Electron Configuration Of Bromine (Br) The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. First, find electrons of bromine atom. Since the atomic number of bromine is 35, the total electrons of bromine are 35. You can also see the image given below; From the electrons in an atom, to the differing. The shorthand electron configuration (or noble gas configuration) as well as. Bromine is in the. What Is The Ground State Electron Configuration Of Bromine (Br).
From app.emaze.com
Bromine ) on emaze What Is The Ground State Electron Configuration Of Bromine (Br) Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Use a chart such as the one below. First, find electrons of bromine atom. Second, make a table of subshell and its maximum electrons. The atomic number of bromine represents the total number of electrons of bromine.. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) The shorthand electron configuration (or noble gas configuration) as well as. This can be shortened to [ar]4s23d104p5. Second, make a table of subshell and its maximum electrons. First, find electrons of bromine atom. Use a chart such as the one below. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The electron configuration. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry What Is The Ground State Electron Configuration Of Bromine (Br) Electron configuration chart of all elements is mentioned in the table below. You can also see the image given below; From the electrons in an atom, to the differing. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Use a chart such as the one below. Second, make. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) The atomic number of bromine represents the total number of electrons of bromine. The shorthand electron configuration (or noble gas configuration) as well as. From the electrons in an atom, to the differing. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. You can also see the image given. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.youtube.com
How to Write the Atomic Orbital Diagram for Bromine (Br) YouTube What Is The Ground State Electron Configuration Of Bromine (Br) The atomic number of bromine represents the total number of electrons of bromine. Second, make a table of subshell and its maximum electrons. You can also see the image given below; The shorthand electron configuration (or noble gas configuration) as well as. First, find electrons of bromine atom. From the electrons in an atom, to the differing. Ground state electron. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.youtube.com
Br electronic configurationHow to write electronic configuration of What Is The Ground State Electron Configuration Of Bromine (Br) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. This can be shortened to [ar]4s23d104p5. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Second, make a table of subshell and its maximum electrons. From the electrons in an atom, to the. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the differing. First, find electrons of bromine atom. This. What Is The Ground State Electron Configuration Of Bromine (Br).
From material-properties.org
Bromine Periodic Table and Atomic Properties What Is The Ground State Electron Configuration Of Bromine (Br) Since the atomic number of bromine is 35, the total electrons of bromine are 35. Use a chart such as the one below. Second, make a table of subshell and its maximum electrons. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. You can also see the image given below; The atomic number of bromine. What Is The Ground State Electron Configuration Of Bromine (Br).
From www.animalia-life.club
Electron Configuration For Bromine What Is The Ground State Electron Configuration Of Bromine (Br) You can also see the image given below; The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The shorthand electron configuration (or noble gas configuration) as well as. Second, make a table of subshell and its maximum electrons. First, find electrons of bromine atom. Since the atomic number of bromine is 35, the total electrons of bromine are 35. The atomic number. What Is The Ground State Electron Configuration Of Bromine (Br).
From brandonkss.github.io
Ground State Electron Configuration Chart What Is The Ground State Electron Configuration Of Bromine (Br) The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The shorthand electron configuration (or noble gas configuration) as well as. First, find electrons of bromine atom. Second, make a table of subshell and its maximum electrons. This can be shortened to [ar]4s23d104p5. Since the atomic number of bromine is 35, the total electrons of bromine are 35. Use a chart such as. What Is The Ground State Electron Configuration Of Bromine (Br).
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? What Is The Ground State Electron Configuration Of Bromine (Br) From the electrons in an atom, to the differing. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. First, find electrons of bromine atom. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. The atomic number of bromine represents the total number. What Is The Ground State Electron Configuration Of Bromine (Br).