Pda Guidelines For Process Validation . This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. Validation of moist heat sterilization processes: Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international.
from mavink.com
This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Validation of moist heat sterilization processes: This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. Generate evidence of consistency • traditional approach (according.
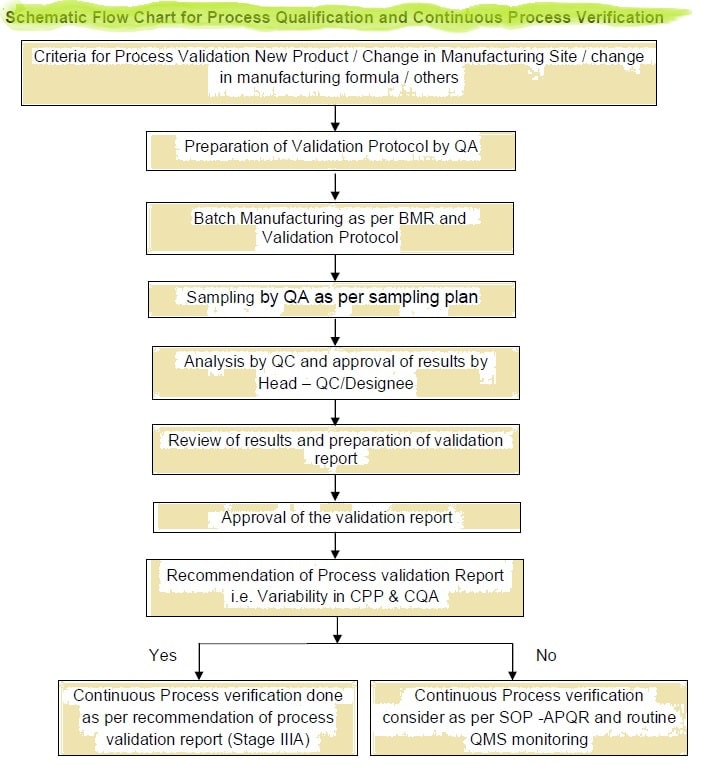
Pharmaceutical Process Validation Flow Chart
Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Validation of moist heat sterilization processes:
From www.scribd.com
Template for Process Validation Protocol Verification And Validation Pda Guidelines For Process Validation This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Validation of moist heat sterilization processes: This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and design of. Pda Guidelines For Process Validation.
From dokumen.tips
(PDF) Guideline on process validation for finished products Pda Guidelines For Process Validation Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities. Pda Guidelines For Process Validation.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Pda Guidelines For Process Validation Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Validation of moist heat sterilization processes: Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This. Pda Guidelines For Process Validation.
From dokumen.tips
(PDF) FDA Process Validation Guideline Stage 3 DOKUMEN.TIPS Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities. Pda Guidelines For Process Validation.
From www.stdlink.com
PDA TR 602 Standard PDF STANDARD PDF SITE Pda Guidelines For Process Validation Cycle design, development, qualification and ongoing. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and design of. Pda Guidelines For Process Validation.
From kneat.com
The Four Types of Process Validation Kneat Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Validation of moist heat sterilization processes: Cycle design, development, qualification and ongoing. This. Pda Guidelines For Process Validation.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Validation of moist heat sterilization processes: This technical report aims to provide clear technical guidance for the development and design of. Pda Guidelines For Process Validation.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Pda Guidelines For Process Validation Validation of moist heat sterilization processes: Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This. Pda Guidelines For Process Validation.
From www.slideserve.com
PPT Current Biopharmaceutical CMC Issues What the PDA is Doing and Pda Guidelines For Process Validation Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and. Pda Guidelines For Process Validation.
From journal.pda.org
PDA Response “Guideline for Submitting Documentation for Sterilization Pda Guidelines For Process Validation Validation of moist heat sterilization processes: Cycle design, development, qualification and ongoing. Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan.. Pda Guidelines For Process Validation.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Validation of moist heat sterilization processes: This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This guidance aligns process validation activities with a product lifecycle concept and with existing. Pda Guidelines For Process Validation.
From www.slideshare.net
Process validation Pda Guidelines For Process Validation Generate evidence of consistency • traditional approach (according. Validation of moist heat sterilization processes: This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. This. Pda Guidelines For Process Validation.
From www.slideserve.com
PPT Process Validation General Principles and Practices PowerPoint Pda Guidelines For Process Validation This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of. Pda Guidelines For Process Validation.
From bmcmedinformdecismak.biomedcentral.com
Development and validation of a patient decision aid for prostate Pda Guidelines For Process Validation Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This guidance aligns process validation activities. Pda Guidelines For Process Validation.
From www.researchgate.net
(PDF) USFDA Guidelines on Process Validation A Review Pda Guidelines For Process Validation Validation of moist heat sterilization processes: Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan.. Pda Guidelines For Process Validation.
From vdocuments.site
Process Validation PDA Technical Report No. 60 as a Technical Pda Guidelines For Process Validation Validation of moist heat sterilization processes: This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Cycle design, development, qualification and ongoing. This. Pda Guidelines For Process Validation.
From orioledhub.eu
The Lifecycle Approach to Process Validation Overview Orioled Hub Pda Guidelines For Process Validation This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Cycle design, development, qualification and ongoing. Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Validation of moist heat sterilization processes: This. Pda Guidelines For Process Validation.
From www.slideserve.com
PPT Process Validation What the Future Holds PowerPoint Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Validation of moist heat sterilization processes: This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of. Pda Guidelines For Process Validation.
From www.collidu.com
Process Validation PowerPoint Presentation Slides PPT Template Pda Guidelines For Process Validation This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Validation of moist heat sterilization processes: Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. This. Pda Guidelines For Process Validation.
From www.researchgate.net
(PDF) PDA Annual Meeting Assessment Methodologies for Process Pda Guidelines For Process Validation This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Validation of moist heat sterilization processes: Cycle design, development, qualification and ongoing. This. Pda Guidelines For Process Validation.
From www.yumpu.com
Process Validation PDA Technical Report No. 60 as a practical Pda Guidelines For Process Validation Validation of moist heat sterilization processes: Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Generate evidence of consistency • traditional approach (according. This. Pda Guidelines For Process Validation.
From mavink.com
Pharmaceutical Process Validation Flow Chart Pda Guidelines For Process Validation This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. Validation of moist heat sterilization processes: This technical report aims to provide clear. Pda Guidelines For Process Validation.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This guidance aligns process validation activities. Pda Guidelines For Process Validation.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Pda Guidelines For Process Validation Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Cycle design, development, qualification and ongoing. Validation of moist heat sterilization processes: This. Pda Guidelines For Process Validation.
From blog.johner-institute.com
Process validation Definition & examples What to look out for Pda Guidelines For Process Validation Cycle design, development, qualification and ongoing. Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Validation of moist heat sterilization processes:. Pda Guidelines For Process Validation.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Pda Guidelines For Process Validation Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and. Pda Guidelines For Process Validation.
From www.youtube.com
Practical Application Points for Process Validation Lifecycle Approach Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Validation of moist heat sterilization processes: Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This guidance aligns process validation activities with a. Pda Guidelines For Process Validation.
From ispe.org
Biopharmaceutical Manufacturing Process Validation and Quality Risk Pda Guidelines For Process Validation This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance for the development and design of. Pda Guidelines For Process Validation.
From fedegari.com
PDAValidation of Moist Heat Sterilization Processes Fedegari Pda Guidelines For Process Validation Validation of moist heat sterilization processes: Cycle design, development, qualification and ongoing. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear technical guidance. Pda Guidelines For Process Validation.
From cmsmedtech.com
Free ISO 13485 Process Validation Template Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. Validation of moist heat sterilization processes: Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This. Pda Guidelines For Process Validation.
From dokumen.tips
(PDF) Process Validation Guidelines · production EMA Pda Guidelines For Process Validation This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. Cycle design, development, qualification and ongoing. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Validation of moist heat sterilization processes: This. Pda Guidelines For Process Validation.
From www.slideshare.net
PROCESS VALIDATION Pda Guidelines For Process Validation Validation of moist heat sterilization processes: This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Cycle design, development, qualification and ongoing. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Generate evidence of consistency • traditional approach (according. This. Pda Guidelines For Process Validation.
From www.semanticscholar.org
[PDF] Fda 2011 Process validation Guidance Process validation Pda Guidelines For Process Validation Validation of moist heat sterilization processes: Cycle design, development, qualification and ongoing. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. Generate evidence of consistency • traditional approach (according. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This. Pda Guidelines For Process Validation.
From www.slideteam.net
MustHave Process Validation Templates with Samples and Examples Pda Guidelines For Process Validation Cycle design, development, qualification and ongoing. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. Generate evidence of consistency • traditional approach (according. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear. Pda Guidelines For Process Validation.
From journal.pda.org
Simplifying and Improving Process Validation PDA Journal of Pda Guidelines For Process Validation Generate evidence of consistency • traditional approach (according. Cycle design, development, qualification and ongoing. This guidance aligns process validation activities with a product lifecycle concept and with existing fda guidance, including the fda/international. This technical report aims to provide clear technical guidance for the development and design of a process validation master plan. This technical report aims to provide clear. Pda Guidelines For Process Validation.