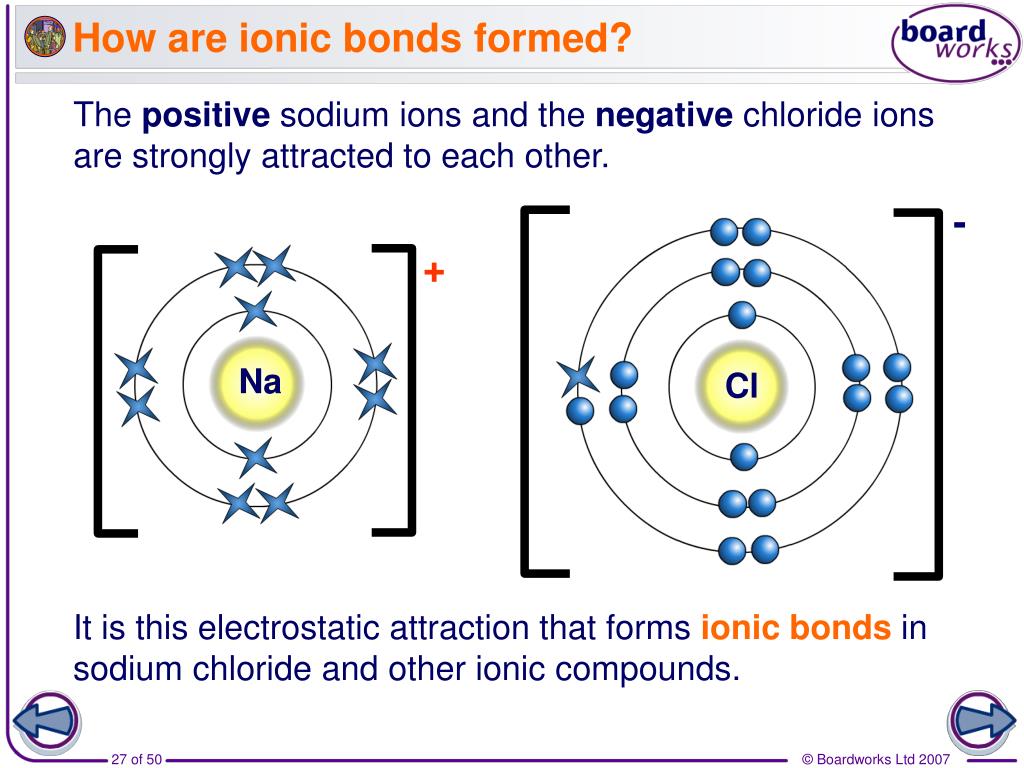
Are Ionic Bonds Metals . Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. Ionic bonds form between a metal and a nonmetal. Covalent bonds form when two nonmetals share electrons in a chemical bond. The resulting compound is called an ionic. Covalent bonds are highly stable bonds with. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Neither atom is strong enough to attract. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. Ionic bonds form when there is a large electronegativity difference between atoms or. The metal donates a valence electron to the nonmetal to form the bond.
from www.slideserve.com
Ionic bonds form between a metal and a nonmetal. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Covalent bonds are highly stable bonds with. The metal donates a valence electron to the nonmetal to form the bond. Ionic bonds form when there is a large electronegativity difference between atoms or. Neither atom is strong enough to attract. The resulting compound is called an ionic. Covalent bonds form when two nonmetals share electrons in a chemical bond.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Are Ionic Bonds Metals By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic bonds form between a metal and a nonmetal. Covalent bonds form when two nonmetals share electrons in a chemical bond. Ionic bonds form when there is a large electronegativity difference between atoms or. Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The resulting compound is called an ionic. Neither atom is strong enough to attract. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. The metal donates a valence electron to the nonmetal to form the bond. Covalent bonds are highly stable bonds with.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Are Ionic Bonds Metals Ionic bonds form between a metal and a nonmetal. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Neither atom is strong enough to attract. Metallic bonds form between metal atoms,. Are Ionic Bonds Metals.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 Are Ionic Bonds Metals By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic bonds form between a metal and a nonmetal. Covalent bonds are highly stable bonds with. The metal donates a valence electron to the nonmetal to form the bond. Ionic compounds tend to be crystalline structures with high melting. Are Ionic Bonds Metals.
From www.slideserve.com
PPT Ionic Bonds PowerPoint Presentation, free download ID1990542 Are Ionic Bonds Metals The metal donates a valence electron to the nonmetal to form the bond. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. Neither atom is strong enough to attract. Ionic compounds tend to be crystalline structures with high melting points. Are Ionic Bonds Metals.
From slideplayer.com
Ionic Bonding. ppt download Are Ionic Bonds Metals Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. The resulting compound is called an ionic. Covalent bonds are highly stable bonds with. Covalent bonds form when two nonmetals share electrons in a chemical bond. Ionic bonds form between a metal and a nonmetal. Ionic bonds form when there is a large electronegativity difference between. Are Ionic Bonds Metals.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Are Ionic Bonds Metals The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. Covalent bonds are highly stable bonds with. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Neither atom is strong enough to attract.. Are Ionic Bonds Metals.
From www.worksheetsplanet.com
What is an Ionic Bond? Are Ionic Bonds Metals Covalent bonds are highly stable bonds with. The resulting compound is called an ionic. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. Ionic bonds form when there is a large electronegativity difference between atoms or. Covalent bonds form when. Are Ionic Bonds Metals.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Are Ionic Bonds Metals Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Ionic bonds form when there is a large electronegativity difference between atoms or. Neither atom is strong enough to attract. Covalent bonds are highly stable bonds with. Ionic bonds form between a metal and a nonmetal. Covalent bonds form when two nonmetals share. Are Ionic Bonds Metals.
From www.slideserve.com
PPT Metallic Bonds & Ionic Bonds PowerPoint Presentation, free Are Ionic Bonds Metals Covalent bonds are highly stable bonds with. Covalent bonds form when two nonmetals share electrons in a chemical bond. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a. Are Ionic Bonds Metals.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Are Ionic Bonds Metals Covalent bonds are highly stable bonds with. Covalent bonds form when two nonmetals share electrons in a chemical bond. The resulting compound is called an ionic. Ionic bonds form when there is a large electronegativity difference between atoms or. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged.. Are Ionic Bonds Metals.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID2962570 Are Ionic Bonds Metals Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. Ionic bonds form between a metal and a nonmetal. The resulting compound is called an ionic. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic bonds form when there is a large electronegativity difference between atoms or. By definition,. Are Ionic Bonds Metals.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Are Ionic Bonds Metals Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The resulting compound is called an ionic. Covalent bonds form when two nonmetals share electrons in a chemical bond. Ionic bonds form when there is a large electronegativity difference between atoms or. Metallic bonds form between metal atoms, where the valence electrons float. Are Ionic Bonds Metals.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Are Ionic Bonds Metals The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. The metal donates a valence electron to the nonmetal to form the bond. Neither atom is strong enough to attract. Covalent bonds form when two nonmetals share electrons in a chemical. Are Ionic Bonds Metals.
From sciencenotes.org
Ionic Bond Definition and Examples Are Ionic Bonds Metals Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. The resulting compound is called an ionic. Ionic bonds form between a metal and. Are Ionic Bonds Metals.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Are Ionic Bonds Metals Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic bonds form when there is a large electronegativity difference between atoms or. Neither atom is strong enough to attract. Ionic bonds. Are Ionic Bonds Metals.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download Are Ionic Bonds Metals By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. The resulting compound is called an ionic. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids. Are Ionic Bonds Metals.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Are Ionic Bonds Metals Covalent bonds are highly stable bonds with. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. The resulting compound is called an ionic. Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. Ionic compounds tend. Are Ionic Bonds Metals.
From www.slideserve.com
PPT CHAPTER 2 A tomic structure and interatomic bonding PowerPoint Are Ionic Bonds Metals Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic bonds form when there is a large electronegativity difference between atoms or. Covalent bonds form when two nonmetals share electrons in a chemical bond. The metal donates a valence electron to the nonmetal to form the bond. Metallic bonds form between metal atoms, where. Are Ionic Bonds Metals.
From learnwithdrscott.com
Ionic Bond Definition Easy Hard Science Are Ionic Bonds Metals Ionic bonds form when there is a large electronegativity difference between atoms or. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. Covalent bonds are highly stable bonds with. The metal donates a valence electron to the nonmetal to form. Are Ionic Bonds Metals.
From sciencenotes.org
Types of Chemical Bonds Are Ionic Bonds Metals Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. Neither atom is strong enough to attract. The metal donates a valence electron to the nonmetal to form the bond. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a. Are Ionic Bonds Metals.
From en.wikipedia.org
Ionic bonding Wikipedia Are Ionic Bonds Metals Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The metal donates a valence electron to the nonmetal to form the bond. Ionic bonds form when there is a large electronegativity difference between atoms or. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell. Are Ionic Bonds Metals.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Are Ionic Bonds Metals Covalent bonds are highly stable bonds with. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The resulting compound is called an ionic. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic bonds form when there is a large. Are Ionic Bonds Metals.
From www.slideserve.com
PPT Ionic and Metallic Bonding PowerPoint Presentation, free download Are Ionic Bonds Metals Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Covalent bonds are highly stable bonds with. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic compounds tend to be crystalline structures with high melting points that are water soluble.. Are Ionic Bonds Metals.
From tuitiontube.com
Ionic Bond and Ionic Bond Formation, Definition, Properties in Are Ionic Bonds Metals Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic bonds form when there is a large electronegativity difference between atoms or. The resulting compound is called an ionic. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Metallic bonds form between. Are Ionic Bonds Metals.
From www2.victoriacollege.edu
formation of ionic bonds Are Ionic Bonds Metals Ionic bonds form when there is a large electronegativity difference between atoms or. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Covalent bonds form when two nonmetals share electrons in a chemical bond. Neither atom is strong enough to attract. Ionic bonds form between a metal and. Are Ionic Bonds Metals.
From www.sliderbase.com
Chemical Bonds Are Ionic Bonds Metals Ionic bonds form when there is a large electronegativity difference between atoms or. The metal donates a valence electron to the nonmetal to form the bond. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic bonds form between a metal and a nonmetal. The bonding within ionic or covalent solids may be stronger,. Are Ionic Bonds Metals.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4982303 Are Ionic Bonds Metals Neither atom is strong enough to attract. Ionic bonds form between a metal and a nonmetal. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Metallic bonds form between metal atoms, where the valence electrons float between multiple atoms. Covalent bonds form when two nonmetals share electrons in a chemical bond. The. Are Ionic Bonds Metals.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Are Ionic Bonds Metals Ionic compounds tend to be crystalline structures with high melting points that are water soluble. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. The resulting compound is called an ionic. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical.. Are Ionic Bonds Metals.
From www.youtube.com
Examples of Ionic Compoiunds YouTube Are Ionic Bonds Metals Covalent bonds form when two nonmetals share electrons in a chemical bond. The resulting compound is called an ionic. Ionic bonds form when there is a large electronegativity difference between atoms or. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. The metal donates a valence electron to. Are Ionic Bonds Metals.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Are Ionic Bonds Metals Neither atom is strong enough to attract. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. The metal donates a valence electron to the nonmetal to form the bond. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject. Are Ionic Bonds Metals.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Are Ionic Bonds Metals The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. Covalent bonds form when two nonmetals share electrons in a chemical bond. Metallic bonds. Are Ionic Bonds Metals.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Bonds Metals By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. Ionic bonds form between a metal and a nonmetal. The metal donates a valence electron to the nonmetal to form the bond. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these. Are Ionic Bonds Metals.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Are Ionic Bonds Metals The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. Ionic bonds form between a metal and a nonmetal. The resulting compound is called an ionic. Neither atom is strong enough to attract. Ionic bond, type of linkage formed from the. Are Ionic Bonds Metals.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Are Ionic Bonds Metals Ionic compounds tend to be crystalline structures with high melting points that are water soluble. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. The resulting compound is called an ionic. Neither atom is strong enough to attract. Covalent bonds are highly stable bonds with. The metal donates. Are Ionic Bonds Metals.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps Are Ionic Bonds Metals Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical. The metal donates a valence electron to the nonmetal to form the bond. Covalent bonds are highly stable bonds with. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Neither atom is strong enough to attract. By. Are Ionic Bonds Metals.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Are Ionic Bonds Metals The resulting compound is called an ionic. Covalent bonds form when two nonmetals share electrons in a chemical bond. The bonding within ionic or covalent solids may be stronger, but it is also directional, making these solids subject to fracture (brittle) when struck with a hammer, for example. Covalent bonds are highly stable bonds with. Metallic bonds form between metal. Are Ionic Bonds Metals.