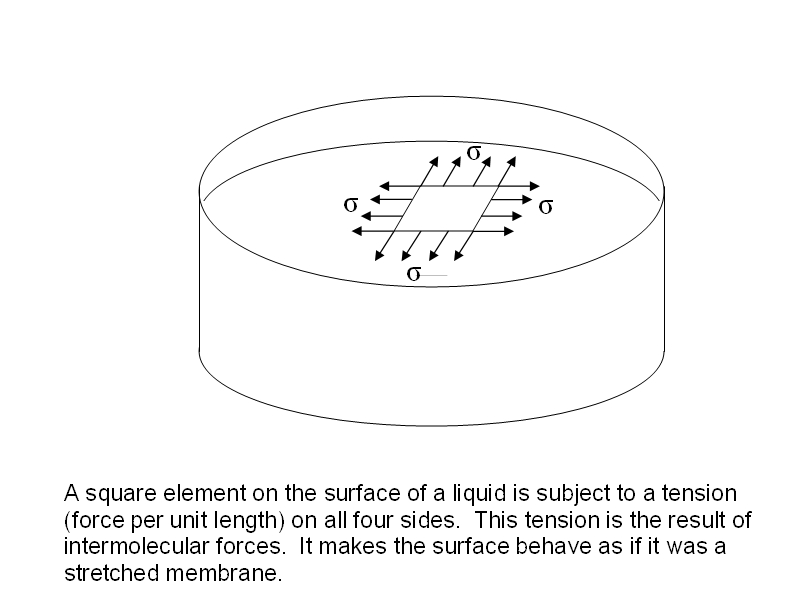
Does Surface Tension Increase With Molar Mass . The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. The strength of intermolecular forces (and therefore impact. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The intermolecular forces increase with increasing polarization of bonds. Besides mercury, water has the highest surface tension for all liquids. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Water's high surface tension is due to the hydrogen bonding in water molecules. To make predictions of surface tension, our method needs accurate experimental data as inputs, including:
from fsz.ifas.ufl.edu
The molecules on the surface of a liquid are attracted by their neighbors from the. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: The strength of intermolecular forces (and therefore impact. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Water's high surface tension is due to the hydrogen bonding in water molecules. Besides mercury, water has the highest surface tension for all liquids. The intermolecular forces increase with increasing polarization of bonds.
Surface Tension
Does Surface Tension Increase With Molar Mass Water's high surface tension is due to the hydrogen bonding in water molecules. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Water's high surface tension is due to the hydrogen bonding in water molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. The molecules on the surface of a liquid are attracted by their neighbors from the. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Besides mercury, water has the highest surface tension for all liquids. The strength of intermolecular forces (and therefore impact. The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The intermolecular forces increase with increasing polarization of bonds.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free download ID6578004 Does Surface Tension Increase With Molar Mass Water's high surface tension is due to the hydrogen bonding in water molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid. Does Surface Tension Increase With Molar Mass.
From www.researchgate.net
Variation in Surface tension with micro molar concentration of... Download Scientific Diagram Does Surface Tension Increase With Molar Mass Water's high surface tension is due to the hydrogen bonding in water molecules. The intermolecular forces increase with increasing polarization of bonds. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is. Does Surface Tension Increase With Molar Mass.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co Does Surface Tension Increase With Molar Mass Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Besides mercury, water has the highest surface tension for all liquids. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension is a measure of the energy required to increase the interface surface area,. Does Surface Tension Increase With Molar Mass.
From www.researchgate.net
Surface tension (γ) versus molar concentration of compound I at 25 • C. Download Scientific Does Surface Tension Increase With Molar Mass Besides mercury, water has the highest surface tension for all liquids. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. The strength of intermolecular forces (and. Does Surface Tension Increase With Molar Mass.
From www.numerade.com
SOLVED How is dipole moment related to heat of vaporization? viscosity? mole fraction? average Does Surface Tension Increase With Molar Mass The molecules on the surface of a liquid are attracted by their neighbors from the. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Water's high surface tension is due to the hydrogen bonding in water molecules. The surface tension is a measure of the energy required to increase the interface surface area, and. Does Surface Tension Increase With Molar Mass.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it impart YouTube Does Surface Tension Increase With Molar Mass Water's high surface tension is due to the hydrogen bonding in water molecules. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. The intermolecular forces increase with increasing polarization of bonds. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid. Does Surface Tension Increase With Molar Mass.
From www.researchgate.net
Comparison of surface tension deviations, Δσ, with excess molar... Download Scientific Diagram Does Surface Tension Increase With Molar Mass The intermolecular forces increase with increasing polarization of bonds. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: The surface tension is a measure of the energy required to increase the interface surface area,. Does Surface Tension Increase With Molar Mass.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Does Surface Tension Increase With Molar Mass The strength of intermolecular forces (and therefore impact. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Besides mercury, water has the highest surface tension for all liquids. The surface tension is a measure of the energy required to increase the interface surface area, and. Does Surface Tension Increase With Molar Mass.
From www.researchgate.net
Variation in Surface tension with micro molar concentration of... Download Scientific Diagram Does Surface Tension Increase With Molar Mass Water's high surface tension is due to the hydrogen bonding in water molecules. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The strength of intermolecular forces (and therefore impact. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Besides mercury, water has the highest surface tension for all liquids.. Does Surface Tension Increase With Molar Mass.
From www.youtube.com
Surface Tension of Water Explained YouTube Does Surface Tension Increase With Molar Mass Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. The molecules on the surface of a liquid are attracted by their neighbors from the. The strength of intermolecular forces (and therefore impact. To make predictions of surface tension, our method needs accurate experimental data as. Does Surface Tension Increase With Molar Mass.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Illustration of science Does Surface Tension Increase With Molar Mass The strength of intermolecular forces (and therefore impact. The intermolecular forces increase with increasing polarization of bonds. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Besides mercury, water has the highest surface tension for all liquids. Surface tension is the energy, or work, required. Does Surface Tension Increase With Molar Mass.
From www.researchgate.net
(PDF) Surface Tension, Density, and Molar Volume of Liquid SbSn Alloys Experiment Versus Modeling Does Surface Tension Increase With Molar Mass The strength of intermolecular forces (and therefore impact. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Surface tension is the energy, or work, required to increase the surface area. Does Surface Tension Increase With Molar Mass.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Does Surface Tension Increase With Molar Mass Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. Water's high surface tension is due to the hydrogen bonding in water molecules. Besides mercury, water has the highest surface tension for all liquids. The intermolecular forces increase with increasing. Does Surface Tension Increase With Molar Mass.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Presentation ID3525649 Does Surface Tension Increase With Molar Mass The intermolecular forces increase with increasing polarization of bonds. The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. The strength of intermolecular forces (and therefore impact. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The molecules on the surface of a. Does Surface Tension Increase With Molar Mass.
From www.vedantu.com
States of Matter Chapter For JEE Main Chemistry Does Surface Tension Increase With Molar Mass Water's high surface tension is due to the hydrogen bonding in water molecules. The molecules on the surface of a liquid are attracted by their neighbors from the. The strength of intermolecular forces (and therefore impact. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension is. Does Surface Tension Increase With Molar Mass.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Does Surface Tension Increase With Molar Mass The intermolecular forces increase with increasing polarization of bonds. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Water's high surface tension is due to the hydrogen bonding in water molecules. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid. Does Surface Tension Increase With Molar Mass.
From chem.libretexts.org
12.2 Some Properties of Liquids Chemistry LibreTexts Does Surface Tension Increase With Molar Mass The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Water's high surface tension is due to the hydrogen bonding in water molecules. The intermolecular forces increase with increasing polarization of bonds.. Does Surface Tension Increase With Molar Mass.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Does Surface Tension Increase With Molar Mass The intermolecular forces increase with increasing polarization of bonds. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water's high surface tension is due to the hydrogen bonding in water molecules. The strength of intermolecular forces (and therefore impact. The molecules on the surface of a liquid are attracted. Does Surface Tension Increase With Molar Mass.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces between water molecules Does Surface Tension Increase With Molar Mass The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Besides mercury, water has the highest surface tension for all liquids. Since these intermolecular forces vary depending. Does Surface Tension Increase With Molar Mass.
From www.slideserve.com
PPT Ch. 2 States of Matter PowerPoint Presentation, free download ID7090893 Does Surface Tension Increase With Molar Mass The strength of intermolecular forces (and therefore impact. Besides mercury, water has the highest surface tension for all liquids. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. The surface tension is a measure of the energy required to increase the interface surface area, and. Does Surface Tension Increase With Molar Mass.
From www.askiitians.com
Colligative Properties and Determination of Molar Mass Study Material for IIT JEE askIITians Does Surface Tension Increase With Molar Mass The strength of intermolecular forces (and therefore impact. The intermolecular forces increase with increasing polarization of bonds. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Water's high surface tension is due to the hydrogen bonding in water molecules. The surface tension is a measure of the energy required to increase the interface surface. Does Surface Tension Increase With Molar Mass.
From www.doubtnut.com
KCl, CH(3)(CH(2))OSO(3)^()Na^(+), CH(3)OH Does Surface Tension Increase With Molar Mass Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Besides mercury, water has the highest surface tension for all liquids. To make predictions of surface. Does Surface Tension Increase With Molar Mass.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Does Surface Tension Increase With Molar Mass To make predictions of surface tension, our method needs accurate experimental data as inputs, including: The molecules on the surface of a liquid are attracted by their neighbors from the. Water's high surface tension is due to the hydrogen bonding in water molecules. The intermolecular forces increase with increasing polarization of bonds. Surface tension is the energy, or work, required. Does Surface Tension Increase With Molar Mass.
From www.researchgate.net
Surface tension values for different types of mixtures versus molar... Download Scientific Diagram Does Surface Tension Increase With Molar Mass Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. Water's high surface tension is due to the hydrogen bonding in water molecules.. Does Surface Tension Increase With Molar Mass.
From mungfali.com
Surface Tension Chart Does Surface Tension Increase With Molar Mass The strength of intermolecular forces (and therefore impact. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The intermolecular. Does Surface Tension Increase With Molar Mass.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Presentation ID3374064 Does Surface Tension Increase With Molar Mass Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water's high surface tension is due to the hydrogen bonding in water molecules. The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. Surface tension is the. Does Surface Tension Increase With Molar Mass.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube Does Surface Tension Increase With Molar Mass The molecules on the surface of a liquid are attracted by their neighbors from the. The strength of intermolecular forces (and therefore impact. Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to. To make predictions of surface tension, our method needs accurate experimental data as. Does Surface Tension Increase With Molar Mass.
From www.youtube.com
Surfactant and Surface Tension in Respiration Breathing Mechanics Respiratory Physiology Does Surface Tension Increase With Molar Mass To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Water's high surface tension is due to the hydrogen bonding in water molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area of. Does Surface Tension Increase With Molar Mass.
From byjus.com
Explain the surface tension phenomenon with examples. Does Surface Tension Increase With Molar Mass Water's high surface tension is due to the hydrogen bonding in water molecules. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The molecules on the surface of a liquid are attracted by their neighbors from the. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: The strength of intermolecular. Does Surface Tension Increase With Molar Mass.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Does Surface Tension Increase With Molar Mass Since these intermolecular forces vary depending on the nature of the liquid (e.g. Besides mercury, water has the highest surface tension for all liquids. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: The surface tension is a measure of the energy required to increase the interface surface area, and it is often used. Does Surface Tension Increase With Molar Mass.
From www.vrogue.co
A Variation Of The Surface Tension And Viscosity Of T vrogue.co Does Surface Tension Increase With Molar Mass Since these intermolecular forces vary depending on the nature of the liquid (e.g. The strength of intermolecular forces (and therefore impact. To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Surface tension is the energy required to increase the surface area of a liquid by a unit amount and varies greatly from liquid to.. Does Surface Tension Increase With Molar Mass.
From www.youtube.com
Derive the relation between the surface tension and surface energy of liquid simplified YouTube Does Surface Tension Increase With Molar Mass To make predictions of surface tension, our method needs accurate experimental data as inputs, including: Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The intermolecular forces increase with increasing polarization of bonds. Water's high surface tension is due to the. Does Surface Tension Increase With Molar Mass.
From www.researchgate.net
Influence of molar fraction of liquid acetone at droplet surface on... Download Scientific Diagram Does Surface Tension Increase With Molar Mass The strength of intermolecular forces (and therefore impact. The molecules on the surface of a liquid are attracted by their neighbors from the. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The surface tension is a measure of the energy. Does Surface Tension Increase With Molar Mass.
From www.researchgate.net
Surface tension versus the logarithm of the aqueous molar concentration... Download Scientific Does Surface Tension Increase With Molar Mass Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The strength of intermolecular forces (and therefore impact. The surface tension is a measure of the energy required to increase the interface surface area, and it is often used as a. To make predictions of surface tension, our method needs accurate experimental data as inputs,. Does Surface Tension Increase With Molar Mass.
From fsz.ifas.ufl.edu
Surface Tension Does Surface Tension Increase With Molar Mass The molecules on the surface of a liquid are attracted by their neighbors from the. The strength of intermolecular forces (and therefore impact. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The intermolecular forces increase with increasing polarization of bonds. Surface tension is a phenomenon that occurs due. Does Surface Tension Increase With Molar Mass.