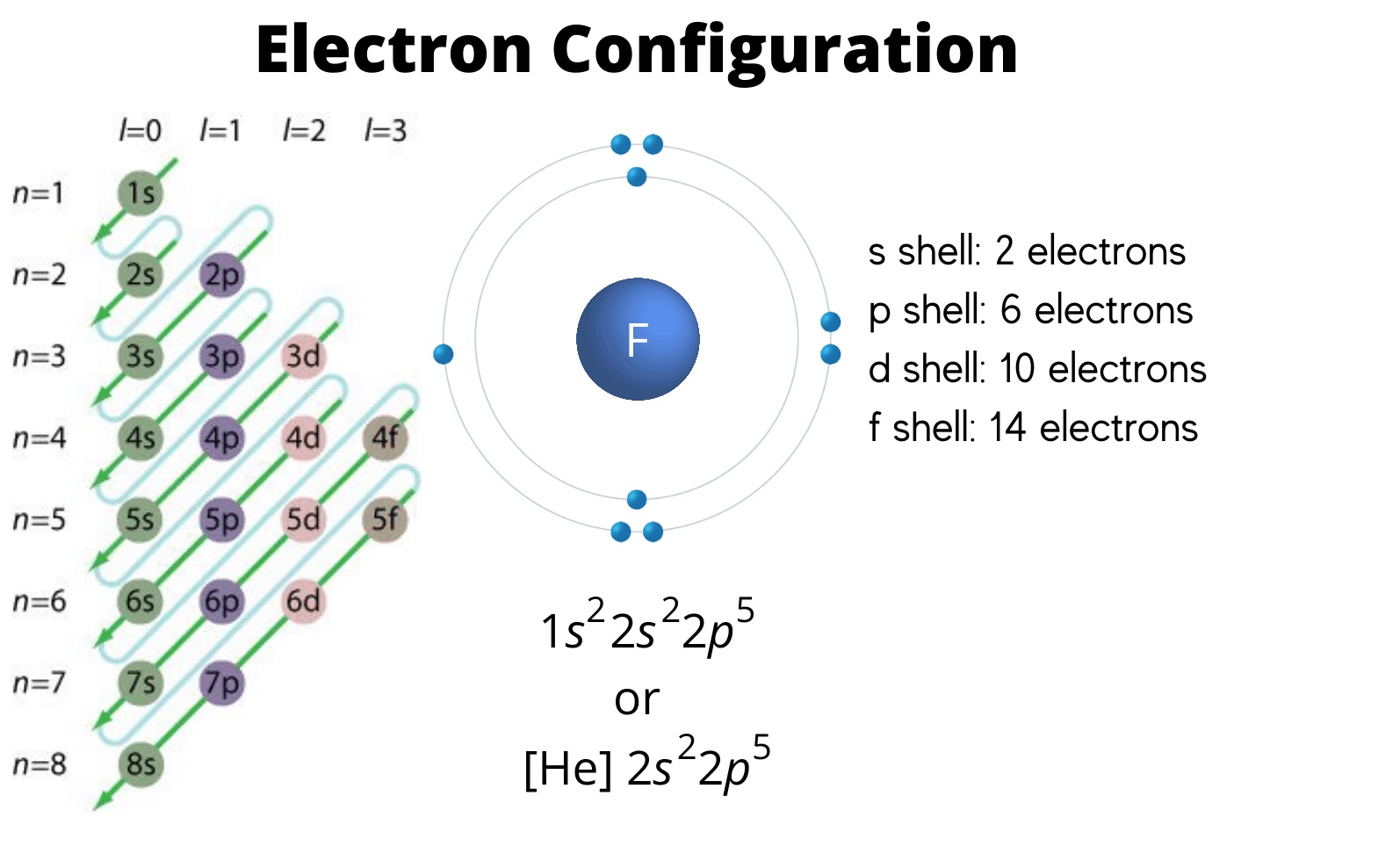
Fe Electron Configuration Short Form . Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. Note that this is not always the same way they. Nitrates and iron chloride are used as industrial reagents. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. The iron sulfate is used in the fungicide. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe.
from facts.net
The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. Nitrates and iron chloride are used as industrial reagents. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The iron sulfate is used in the fungicide. The shorthand electron configuration (or noble gas configuration) as well as. Note that this is not always the same way they. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Electron configuration chart of all elements is mentioned in the table below. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals.
10 Surprising Facts About Electron Configuration Notation
Fe Electron Configuration Short Form The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Note that this is not always the same way they. The shorthand electron configuration (or noble gas configuration) as well as. Nitrates and iron chloride are used as industrial reagents. Electron configuration chart of all elements is mentioned in the table below. The iron sulfate is used in the fungicide. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds.
From ar.inspiredpencil.com
Electron Configuration Of Iron Fe Electron Configuration Short Form Nitrates and iron chloride are used as industrial reagents. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. The iron sulfate is used in the fungicide. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration. Fe Electron Configuration Short Form.
From www.youtube.com
Short form electron configuration YouTube Fe Electron Configuration Short Form The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. Nitrates and iron chloride are used as industrial reagents.. Fe Electron Configuration Short Form.
From www.numerade.com
SOLVED18. Write out the electron configuration for Fe" , Fe'? and Fe Fe Electron Configuration Short Form Electron configuration chart of all elements is mentioned in the table below. The iron sulfate is used in the fungicide. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. The shorthand electron configuration (or noble gas configuration) as well as. First you. Fe Electron Configuration Short Form.
From preparatorychemistry.com
Abbreviated Electron configurations Fe Electron Configuration Short Form First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Electron configuration chart of all elements is mentioned in the table below. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. The electron configuration for. Fe Electron Configuration Short Form.
From modernizemodest1712.blogspot.com
40 orbital diagram for fe Diagram For You Fe Electron Configuration Short Form The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Nitrates and iron chloride are used as industrial reagents. Note that this is not always the same way they. The iron sulfate is used. Fe Electron Configuration Short Form.
From matias-has-villanueva.blogspot.com
Use Noblegas Notation to Describe the Electron Configurations of Kr Fe Electron Configuration Short Form Nitrates and iron chloride are used as industrial reagents. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Note that this is not always the same way they. The iron sulfate is used in the fungicide. The atomic number of fe is 26, which means that its atoms. Fe Electron Configuration Short Form.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Fe Electron Configuration Short Form The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. Nitrates and iron chloride are used as industrial reagents. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. The. Fe Electron Configuration Short Form.
From www.chegg.com
Solved What is the electron configuration of the Iron(III) Fe Electron Configuration Short Form First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Nitrates and iron chloride are used as industrial reagents. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. Note. Fe Electron Configuration Short Form.
From www.toppr.com
Electronic Configuration of Iron Fe element Valency, Applications Fe Electron Configuration Short Form Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. The iron sulfate is used in the fungicide. Nitrates and iron chloride are used as industrial reagents. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if. Fe Electron Configuration Short Form.
From mungfali.com
PD Electron Configuration Fe Electron Configuration Short Form The iron sulfate is used in the fungicide. Note that this is not always the same way they. Electron configuration chart of all elements is mentioned in the table below. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. Nitrates and iron chloride are used. Fe Electron Configuration Short Form.
From learningauttranadhie1h.z21.web.core.windows.net
Orbital Diagrams And Electron Configuration Worksheets Fe Electron Configuration Short Form Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Note that this is not always the same way they. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Nitrates and iron chloride. Fe Electron Configuration Short Form.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID Fe Electron Configuration Short Form Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Electron configuration chart of all elements is mentioned in the table below. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6. Fe Electron Configuration Short Form.
From www.vedantu.com
Fe{{F}_{6}}]}^{3}} has Fe atom _________hybridized with unpaired Fe Electron Configuration Short Form First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Note that this is not always the same way they. Electron configuration chart of all elements is mentioned in the table below. The electron configuration for any chemical element is basically the process that defines the electrons. Fe Electron Configuration Short Form.
From www.echemi.com
Which monatomic ion has a charge of 1+ and the electron configuration Fe Electron Configuration Short Form The iron sulfate is used in the fungicide. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. Note that this is not always the same way they. The shorthand electron configuration (or noble gas configuration) as well as. Nitrates and iron chloride are used as. Fe Electron Configuration Short Form.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Fe Electron Configuration Short Form Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Electron configuration chart of all elements is mentioned in the table below. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Nitrates and iron chloride are used as industrial reagents. The atomic number of fe is. Fe Electron Configuration Short Form.
From slidesharetrick.blogspot.com
Electron Configuration For K slidesharetrick Fe Electron Configuration Short Form Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+. Fe Electron Configuration Short Form.
From electronschematic.blogspot.com
Electron Schematic What Is The Longhand Electron Configuration For Iron Fe Electron Configuration Short Form The iron sulfate is used in the fungicide. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. Nitrates and iron chloride are used as industrial reagents. The shorthand electron configuration (or noble gas configuration) as well as. Note that this is not always the same. Fe Electron Configuration Short Form.
From newlasertagatlanta.blogspot.com
42 fe2+ orbital diagram Wiring Diagrams Manual Fe Electron Configuration Short Form The iron sulfate is used in the fungicide. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The shorthand electron configuration (or noble gas configuration) as well as. The electronic configuration of fe2+. Fe Electron Configuration Short Form.
From studylib.net
ELECTRON CONFIGURATION (SHORT FORM) n of electrons in Fe Electron Configuration Short Form Note that this is not always the same way they. The iron sulfate is used in the fungicide. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Electron configuration chart of all elements is mentioned in the table below. The electron configuration for any chemical element. Fe Electron Configuration Short Form.
From wirepartcavalierly.z21.web.core.windows.net
Determine The Electron Configuration Of Fe3+ Fe Electron Configuration Short Form The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. Electron configuration chart of all elements is mentioned in the table below. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5.. Fe Electron Configuration Short Form.
From proper-cooking.info
Argon Electron Configuration Shorthand Fe Electron Configuration Short Form Note that this is not always the same way they. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Electron configuration chart of all elements is mentioned in the table below. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron. Fe Electron Configuration Short Form.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write Fe Electron Configuration Short Form The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. The iron sulfate is used in the fungicide. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2. Fe Electron Configuration Short Form.
From fr.wikihow.com
4 manières de écrire la configuration électronique des atomes Fe Electron Configuration Short Form Electron configuration chart of all elements is mentioned in the table below. Note that this is not always the same way they. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. First you should write their normal electron configuration and then when you remove electrons you have to. Fe Electron Configuration Short Form.
From www.youtube.com
Iron (Fe) Electron Configuration YouTube Fe Electron Configuration Short Form Note that this is not always the same way they. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Nitrates and iron chloride are used as industrial reagents. The electronic configuration of fe2+ is. Fe Electron Configuration Short Form.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Fe Electron Configuration Short Form The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. Electron configuration chart of all elements is mentioned in the. Fe Electron Configuration Short Form.
From homedeso.vercel.app
Group 1 Periodic Table Electron Configuration Fe Electron Configuration Short Form The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. Electron configuration chart of all elements is mentioned in the table below. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Note that this. Fe Electron Configuration Short Form.
From ibmole.com
2 +12. Atomic structure Fe Electron Configuration Short Form The shorthand electron configuration (or noble gas configuration) as well as. The iron sulfate is used in the fungicide. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6. Fe Electron Configuration Short Form.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Fe Electron Configuration Short Form Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5.. Fe Electron Configuration Short Form.
From facts.net
10 Surprising Facts About Electron Configuration Notation Fe Electron Configuration Short Form The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. Note that this is not always the same way they. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic. Fe Electron Configuration Short Form.
From animalia-life.club
Electron Configuration Of Arsenic Fe Electron Configuration Short Form The atomic number of fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. First you should write their normal electron configuration and then when you remove electrons you have to take them from the. Fe Electron Configuration Short Form.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts Fe Electron Configuration Short Form Nitrates and iron chloride are used as industrial reagents. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Note that this is not always the same way they. The iron sulfate is used in the fungicide. The shorthand electron configuration (or noble gas configuration) as well as. The atomic number of fe is 26, which means that. Fe Electron Configuration Short Form.
From www.youtube.com
Writing Condensed/Abbreviated Electron Configurations YouTube Fe Electron Configuration Short Form Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. Nitrates and iron chloride are used as industrial reagents. The atomic number of fe is 26, which means that its atoms contain 26 protons. Fe Electron Configuration Short Form.
From atonce.com
The Power of Short Form Boost Your Contents Reach in 2024 Fe Electron Configuration Short Form The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Note that this is not always the same way they. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. Electron configuration chart of all. Fe Electron Configuration Short Form.
From www.youtube.com
Fe^2+ ,Fe^3+ and Fe Electron Configuration (Two Ways)Crash Course Fe Electron Configuration Short Form The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell. The shorthand electron configuration (or noble gas configuration) as well as. Fe2+ contains. Fe Electron Configuration Short Form.
From valenceelectrons.com
Iron(Fe) electron configuration and orbital diagram Fe Electron Configuration Short Form Electron configuration chart of all elements is mentioned in the table below. Note that this is not always the same way they. The shorthand electron configuration (or noble gas configuration) as well as. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Nitrates and iron chloride are used as industrial reagents. The electronic configuration of fe2+ is. Fe Electron Configuration Short Form.