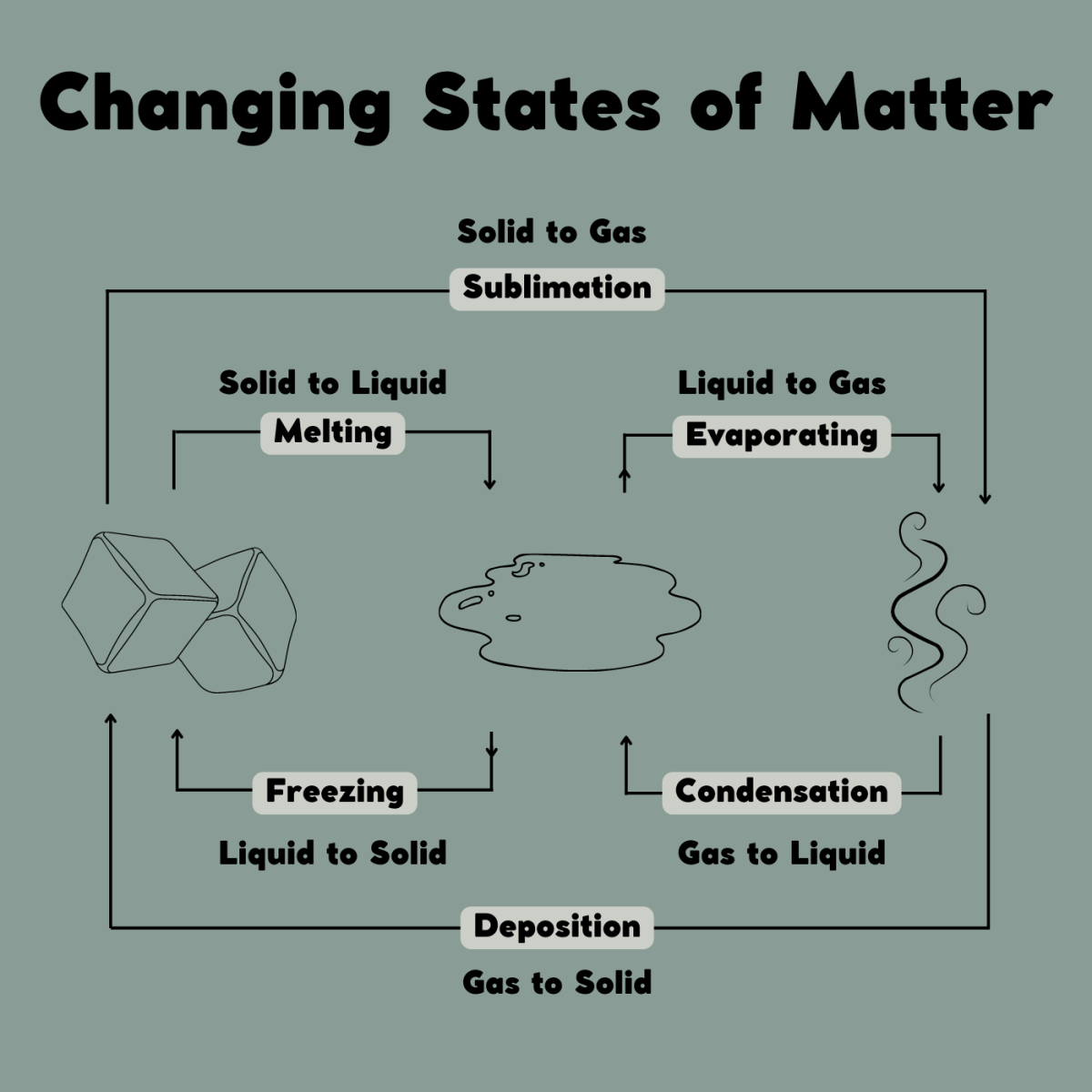
Solid Changes To Liquid Energy . Liquids can vaporize into gases or freeze into solids. We take advantage of changes. We take advantage of changes. Melting, freezing, evaporating and condensing. Solids form by deposition from gases or freezing of liquids. To calculate the energy changes that accompany phase changes. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. Solids can melt into liquids or sublime into gases. Gibbs energy (g¯ g ¯) as a function of temperature (t t). There are four main changes of state: Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. To calculate the energy changes that accompany phase changes. The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling).
from owlcation.com
We take advantage of changes. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. Melting, freezing, evaporating and condensing. We take advantage of changes. Solids form by deposition from gases or freezing of liquids. Solids can melt into liquids or sublime into gases. Liquids can vaporize into gases or freeze into solids. To calculate the energy changes that accompany phase changes. There are four main changes of state: Gibbs energy (g¯ g ¯) as a function of temperature (t t).
What Is the Particle Model? A Guide to Solids, Liquids and Gases Owlcation
Solid Changes To Liquid Energy We take advantage of changes. Melting, freezing, evaporating and condensing. Gibbs energy (g¯ g ¯) as a function of temperature (t t). We take advantage of changes. The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). To calculate the energy changes that accompany phase changes. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. We take advantage of changes. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. Solids can melt into liquids or sublime into gases. There are four main changes of state: Solids form by deposition from gases or freezing of liquids. To calculate the energy changes that accompany phase changes. Liquids can vaporize into gases or freeze into solids.
From saylordotorg.github.io
Solids and Liquids Solid Changes To Liquid Energy There are four main changes of state: To calculate the energy changes that accompany phase changes. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. Solids form by deposition from gases or freezing of liquids. The following plot shows the gibbs energy as a function of temperature, including phase. Solid Changes To Liquid Energy.
From www.ase.org.uk
Solids, liquids and gases Solid Changes To Liquid Energy Liquids can vaporize into gases or freeze into solids. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). After gaining sufficient energy, the molecules. Solid Changes To Liquid Energy.
From www.britannica.com
phase Definition & Facts Britannica Solid Changes To Liquid Energy Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. Gibbs energy (g¯ g ¯) as a function of temperature (t t). To calculate the energy changes that accompany phase changes. Liquids can vaporize into gases or freeze into solids. We take advantage of changes. The following plot shows the. Solid Changes To Liquid Energy.
From maxim-kwise.blogspot.com
The Process by Which a Gas Changes Into a Liquid Solid Changes To Liquid Energy Melting, freezing, evaporating and condensing. Gibbs energy (g¯ g ¯) as a function of temperature (t t). The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). We take advantage of changes. We take advantage of changes. Solids can melt into liquids or sublime into. Solid Changes To Liquid Energy.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Solid Changes To Liquid Energy To calculate the energy changes that accompany phase changes. We take advantage of changes. Melting, freezing, evaporating and condensing. Solids can melt into liquids or sublime into gases. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. To calculate the energy changes that accompany phase changes. We take advantage. Solid Changes To Liquid Energy.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Solid Changes To Liquid Energy To calculate the energy changes that accompany phase changes. We take advantage of changes. Solids form by deposition from gases or freezing of liquids. Solids can melt into liquids or sublime into gases. Melting, freezing, evaporating and condensing. The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid. Solid Changes To Liquid Energy.
From socratic.org
What happens to the molecules in matter when you raise the temperature? Socratic Solid Changes To Liquid Energy Solids can melt into liquids or sublime into gases. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. Solids form by deposition from gases or freezing of liquids. Liquids can vaporize into gases or freeze into solids. We take advantage of changes. The following plot shows the gibbs energy as a function. Solid Changes To Liquid Energy.
From kentchemistry.com
Phase Changes Solid Changes To Liquid Energy Solids form by deposition from gases or freezing of liquids. Melting, freezing, evaporating and condensing. We take advantage of changes. There are four main changes of state: To calculate the energy changes that accompany phase changes. We take advantage of changes. Liquids can vaporize into gases or freeze into solids. After gaining sufficient energy, the molecules of ice escape out. Solid Changes To Liquid Energy.
From emedia.rmit.edu.au
States of matter Learning Lab Solid Changes To Liquid Energy To calculate the energy changes that accompany phase changes. Liquids can vaporize into gases or freeze into solids. Gibbs energy (g¯ g ¯) as a function of temperature (t t). Melting, freezing, evaporating and condensing. To calculate the energy changes that accompany phase changes. Solids can melt into liquids or sublime into gases. After gaining sufficient energy, the molecules of. Solid Changes To Liquid Energy.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement and energy of the Solid Changes To Liquid Energy Melting, freezing, evaporating and condensing. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. There are four main changes of state: To calculate the energy changes that accompany phase changes. To calculate. Solid Changes To Liquid Energy.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in each of the three states of Solid Changes To Liquid Energy Melting, freezing, evaporating and condensing. We take advantage of changes. Solids can melt into liquids or sublime into gases. There are four main changes of state: The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). Although the g g curve is continuous, its first. Solid Changes To Liquid Energy.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet Solid Changes To Liquid Energy To calculate the energy changes that accompany phase changes. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. Gibbs energy (g¯ g ¯) as a function of temperature (t t). To calculate the energy changes that accompany phase changes. Liquids can vaporize into gases or freeze into solids. The following plot shows. Solid Changes To Liquid Energy.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Solid Changes To Liquid Energy Gibbs energy (g¯ g ¯) as a function of temperature (t t). The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). There are four main changes of state: We take advantage of changes. Liquids can vaporize into gases or freeze into solids. To calculate. Solid Changes To Liquid Energy.
From www.britannica.com
phase Definition & Facts Britannica Solid Changes To Liquid Energy We take advantage of changes. Gibbs energy (g¯ g ¯) as a function of temperature (t t). After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. To calculate the energy changes that accompany phase changes. Liquids can vaporize into gases or freeze into solids. There are four main changes of state: To. Solid Changes To Liquid Energy.
From www.snexplores.org
Explainer What are the different states of matter? Solid Changes To Liquid Energy The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). We take advantage of changes. Liquids can vaporize into gases or freeze into solids. Solids can melt into liquids or sublime into gases. After gaining sufficient energy, the molecules of ice escape out of the. Solid Changes To Liquid Energy.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Solid Changes To Liquid Energy Liquids can vaporize into gases or freeze into solids. Solids form by deposition from gases or freezing of liquids. Solids can melt into liquids or sublime into gases. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. Gibbs energy (g¯ g ¯) as a function of temperature (t t).. Solid Changes To Liquid Energy.
From wirelistlatinised.z21.web.core.windows.net
Solid Liquid Gas Diagram Solid Changes To Liquid Energy The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). We take advantage of changes. Melting, freezing, evaporating and condensing. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. There are four main changes. Solid Changes To Liquid Energy.
From kids.britannica.com
solid Students Britannica Kids Homework Help Solid Changes To Liquid Energy We take advantage of changes. To calculate the energy changes that accompany phase changes. We take advantage of changes. There are four main changes of state: Gibbs energy (g¯ g ¯) as a function of temperature (t t). Melting, freezing, evaporating and condensing. Solids can melt into liquids or sublime into gases. Although the g g curve is continuous, its. Solid Changes To Liquid Energy.
From www.slideserve.com
PPT Matter PowerPoint Presentation, free download ID2784659 Solid Changes To Liquid Energy Gibbs energy (g¯ g ¯) as a function of temperature (t t). Solids can melt into liquids or sublime into gases. There are four main changes of state: After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. Melting, freezing, evaporating and condensing. To calculate the energy changes that accompany phase changes. The. Solid Changes To Liquid Energy.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid Changes To Liquid Energy Solids form by deposition from gases or freezing of liquids. We take advantage of changes. There are four main changes of state: After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. We take advantage of changes. Solids can melt into liquids or sublime into gases. Liquids can vaporize into gases or freeze. Solid Changes To Liquid Energy.
From www.slideserve.com
PPT PHASE CHANGES PowerPoint Presentation, free download ID5222838 Solid Changes To Liquid Energy Solids can melt into liquids or sublime into gases. Melting, freezing, evaporating and condensing. Liquids can vaporize into gases or freeze into solids. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. We take advantage of changes. Solids form by deposition from gases or freezing of liquids. We take. Solid Changes To Liquid Energy.
From spmscience.blog.onlinetuition.com.my
Changes of State of Matter and Energy SPM Science Solid Changes To Liquid Energy We take advantage of changes. Solids form by deposition from gases or freezing of liquids. To calculate the energy changes that accompany phase changes. To calculate the energy changes that accompany phase changes. The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). Solids can. Solid Changes To Liquid Energy.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation, condensation, sublimation Solid Changes To Liquid Energy To calculate the energy changes that accompany phase changes. Liquids can vaporize into gases or freeze into solids. Gibbs energy (g¯ g ¯) as a function of temperature (t t). Melting, freezing, evaporating and condensing. There are four main changes of state: Solids form by deposition from gases or freezing of liquids. To calculate the energy changes that accompany phase. Solid Changes To Liquid Energy.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid Changes To Liquid Energy Solids can melt into liquids or sublime into gases. Melting, freezing, evaporating and condensing. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. To calculate the energy changes that accompany phase changes.. Solid Changes To Liquid Energy.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid Changes To Liquid Energy We take advantage of changes. Solids can melt into liquids or sublime into gases. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. To calculate the energy changes that accompany phase changes. To calculate the energy changes that accompany phase changes. Solids form by deposition from gases or freezing of liquids. Gibbs. Solid Changes To Liquid Energy.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid Changes To Liquid Energy Gibbs energy (g¯ g ¯) as a function of temperature (t t). To calculate the energy changes that accompany phase changes. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid. Solid Changes To Liquid Energy.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes PowerPoint Presentation ID1445725 Solid Changes To Liquid Energy There are four main changes of state: Solids can melt into liquids or sublime into gases. We take advantage of changes. Gibbs energy (g¯ g ¯) as a function of temperature (t t). To calculate the energy changes that accompany phase changes. Liquids can vaporize into gases or freeze into solids. Melting, freezing, evaporating and condensing. After gaining sufficient energy,. Solid Changes To Liquid Energy.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Illustration Solid Changes To Liquid Energy To calculate the energy changes that accompany phase changes. Solids can melt into liquids or sublime into gases. Gibbs energy (g¯ g ¯) as a function of temperature (t t). After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. There are four main changes of state: We take advantage of changes. We. Solid Changes To Liquid Energy.
From www.vecteezy.com
Three different States of matter solid, liquid and gasuas state. Inter change of state of matter Solid Changes To Liquid Energy There are four main changes of state: Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. Melting, freezing, evaporating and condensing. Solids form by deposition from gases or freezing of liquids. To calculate the energy changes that accompany phase changes. We take advantage of changes. Gibbs energy (g¯ g. Solid Changes To Liquid Energy.
From courses.lumenlearning.com
Phase Changes Boundless Chemistry Solid Changes To Liquid Energy The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). There are four main changes of state: Melting, freezing, evaporating and condensing. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. Solids form by deposition from gases. Solid Changes To Liquid Energy.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid Changes To Liquid Energy Solids form by deposition from gases or freezing of liquids. Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. Liquids can vaporize into gases or freeze into solids. We take advantage of changes. Gibbs energy (g¯ g ¯) as a function of temperature (t t). To calculate the energy. Solid Changes To Liquid Energy.
From guidelistbaquantising.z13.web.core.windows.net
Venn Diagram For Solids Liquids And Gases Solid Changes To Liquid Energy There are four main changes of state: The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). Liquids can vaporize into gases or freeze into solids. We take advantage of changes. Melting, freezing, evaporating and condensing. We take advantage of changes. After gaining sufficient energy,. Solid Changes To Liquid Energy.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases Owlcation Solid Changes To Liquid Energy Liquids can vaporize into gases or freeze into solids. To calculate the energy changes that accompany phase changes. Gibbs energy (g¯ g ¯) as a function of temperature (t t). Although the g g curve is continuous, its first order derivatives (−s − s) is discontinuous at the phase changes. Solids can melt into liquids or sublime into gases. We. Solid Changes To Liquid Energy.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid Changes To Liquid Energy Liquids can vaporize into gases or freeze into solids. We take advantage of changes. There are four main changes of state: Solids form by deposition from gases or freezing of liquids. Solids can melt into liquids or sublime into gases. We take advantage of changes. After gaining sufficient energy, the molecules of ice escape out of the ice cube and. Solid Changes To Liquid Energy.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to temperature. Vector Illustration Solid Changes To Liquid Energy We take advantage of changes. Melting, freezing, evaporating and condensing. After gaining sufficient energy, the molecules of ice escape out of the ice cube and into the. To calculate the energy changes that accompany phase changes. There are four main changes of state: Solids can melt into liquids or sublime into gases. We take advantage of changes. Gibbs energy (g¯. Solid Changes To Liquid Energy.