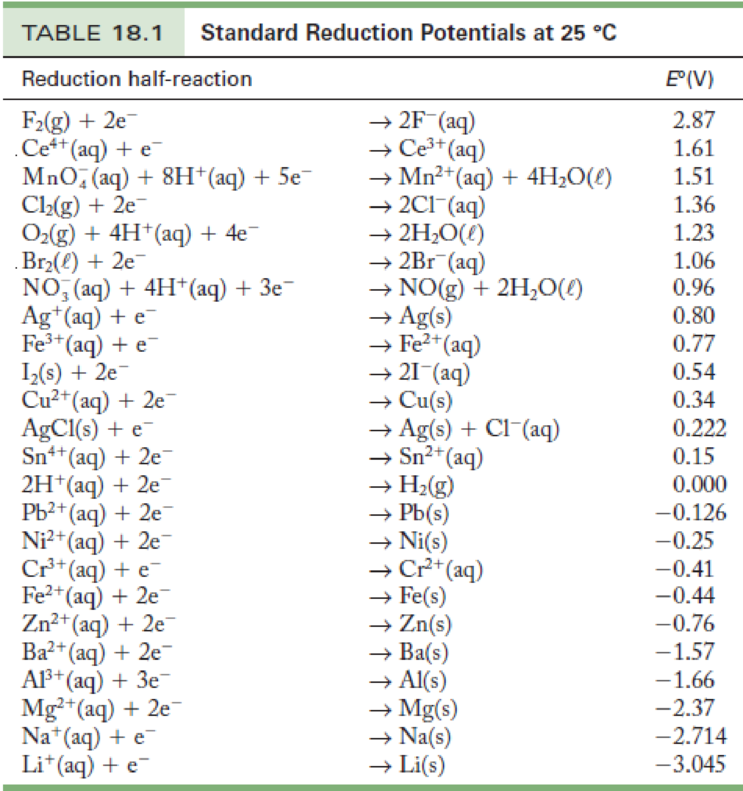
Standard Potential Electrode Reaction . Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Detailed explanation of standard electrode potential provided here. Since the potential of the s.h.e. In an electrochemical cell, an electric potential is created between two dissimilar metals. Standard electrode potential is the potential that arises in a cell under standard conditions.
from dinorahiu-images.blogspot.com
Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Detailed explanation of standard electrode potential provided here. Since the potential of the s.h.e. Standard electrode potential is the potential that arises in a cell under standard conditions. In an electrochemical cell, an electric potential is created between two dissimilar metals.
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint
Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Standard electrode potential is the potential that arises in a cell under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Since the potential of the s.h.e. Detailed explanation of standard electrode potential provided here. In an electrochemical cell, an electric potential is created between two dissimilar metals.
From facts.net
16 Unbelievable Facts About Standard Electrode Potential Standard Potential Electrode Reaction Standard electrode potential is the potential that arises in a cell under standard conditions. In an electrochemical cell, an electric potential is created between two dissimilar metals. Describe and relate the definitions of electrode and cell potentials. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials. Standard Potential Electrode Reaction.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Standard Potential Electrode Reaction Since the potential of the s.h.e. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Detailed explanation of standard electrode potential provided here. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. In an electrochemical cell, an electric. Standard Potential Electrode Reaction.
From mungfali.com
Electrode Potential Series Standard Potential Electrode Reaction 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode potential is the potential that arises in a cell under standard conditions. Interpret electrode potentials in terms of relative oxidant and reductant. Detailed explanation of standard electrode potential provided here. In an electrochemical cell, an electric potential is. Standard Potential Electrode Reaction.
From www.researchgate.net
1 Pathway of a general electrode reaction. Download Scientific Diagram Standard Potential Electrode Reaction Standard electrode potential is the potential that arises in a cell under standard conditions. In an electrochemical cell, an electric potential is created between two dissimilar metals. Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Since the potential. Standard Potential Electrode Reaction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. Standard electrode potential is the potential that arises in a cell under standard conditions. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. In an electrochemical cell, an electric potential is created between two dissimilar metals. Since the potential of the. Standard Potential Electrode Reaction.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Potential Electrode Reaction 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Detailed explanation of standard electrode potential provided here. Standard electrode potential is the potential that arises in a cell under standard conditions. In an electrochemical cell, an electric potential is created between two dissimilar metals. Describe and relate the definitions. Standard Potential Electrode Reaction.
From saylordotorg.github.io
Standard Potentials Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. Since the potential of the s.h.e. Describe and relate the definitions of electrode and cell potentials. In an electrochemical cell, an electric potential is created between two dissimilar metals. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode potential. Standard Potential Electrode Reaction.
From www.slideserve.com
PPT Electrochemical Cells Voltage (Electric potential) PowerPoint Standard Potential Electrode Reaction 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode and cell potentials. Since the potential of the s.h.e. In an electrochemical cell, an electric potential is created between two dissimilar metals. Standard electrode potential is the potential that arises in a cell. Standard Potential Electrode Reaction.
From www.youtube.com
19.1 Standard electrode potential (HL) YouTube Standard Potential Electrode Reaction Since the potential of the s.h.e. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode potential is the potential that arises in a cell under standard conditions. Interpret electrode potentials in terms of relative oxidant and reductant. Detailed explanation of standard electrode potential provided here. Describe and. Standard Potential Electrode Reaction.
From byjus.com
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe Standard Potential Electrode Reaction Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode and cell potentials. Detailed explanation of standard electrode potential provided here. Since the potential of the s.h.e. Standard electrode potential is the potential. Standard Potential Electrode Reaction.
From www.toppr.com
Using the standard electrode potentials given in the table, predict the Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. In an electrochemical cell, an electric potential is created between two dissimilar metals. Since the potential of the s.h.e. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative. Standard Potential Electrode Reaction.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Potential Electrode Reaction Interpret electrode potentials in terms of relative oxidant and reductant. In an electrochemical cell, an electric potential is created between two dissimilar metals. Detailed explanation of standard electrode potential provided here. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Since the potential of the s.h.e. Standard electrode potential. Standard Potential Electrode Reaction.
From www.researchgate.net
Pathway of a general electrode reaction (see [27]). Download Standard Potential Electrode Reaction Since the potential of the s.h.e. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. In an electrochemical cell, an electric potential is created between two dissimilar metals. Standard. Standard Potential Electrode Reaction.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Potential Electrode Reaction Standard electrode potential is the potential that arises in a cell under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Since the potential of the s.h.e. Interpret electrode potentials in terms of relative oxidant and reductant. In an electrochemical cell, an electric potential is created between. Standard Potential Electrode Reaction.
From app.pandai.org
Determine oxidising agent and reducing agent based on value of standard Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. Standard electrode potential is the potential that arises in a cell under standard conditions. Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode and. Standard Potential Electrode Reaction.
From www.scribd.com
Standard Electrode Potentials A Comprehensive Table of Redox Reactions Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. In an electrochemical cell, an electric potential is created between two dissimilar metals. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative oxidant and reductant. Standard electrode potential is the potential that arises in. Standard Potential Electrode Reaction.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Potential Electrode Reaction Since the potential of the s.h.e. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In an electrochemical cell, an electric potential is created between two dissimilar metals. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Detailed. Standard Potential Electrode Reaction.
From www.scribd.com
Standard Electrode Potentials Presentation PDF Chemical Reactions Standard Potential Electrode Reaction Standard electrode potential is the potential that arises in a cell under standard conditions. Since the potential of the s.h.e. In an electrochemical cell, an electric potential is created between two dissimilar metals. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode. Standard Potential Electrode Reaction.
From www.substech.com
standard_electrode_potential.png [SubsTech] Standard Potential Electrode Reaction 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Since the potential of the s.h.e. In an electrochemical cell, an electric potential is created between two dissimilar metals. Standard electrode potential is the potential that arises in a cell under standard conditions. Interpret electrode potentials in terms of relative. Standard Potential Electrode Reaction.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Potential Electrode Reaction Since the potential of the s.h.e. Standard electrode potential is the potential that arises in a cell under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant.. Standard Potential Electrode Reaction.
From www.researchgate.net
Schematic diagram of the electrode reactions for the electrodes with Standard Potential Electrode Reaction In an electrochemical cell, an electric potential is created between two dissimilar metals. Since the potential of the s.h.e. Detailed explanation of standard electrode potential provided here. Interpret electrode potentials in terms of relative oxidant and reductant. Standard electrode potential is the potential that arises in a cell under standard conditions. Describe and relate the definitions of electrode and cell. Standard Potential Electrode Reaction.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. In an electrochemical cell, an electric potential is created between two dissimilar metals. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Since the potential of the s.h.e. Describe and relate the definitions of electrode and cell potentials. Standard electrode potential. Standard Potential Electrode Reaction.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Potential Electrode Reaction In an electrochemical cell, an electric potential is created between two dissimilar metals. Since the potential of the s.h.e. Interpret electrode potentials in terms of relative oxidant and reductant. Detailed explanation of standard electrode potential provided here. Describe and relate the definitions of electrode and cell potentials. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative. Standard Potential Electrode Reaction.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Potential Electrode Reaction In an electrochemical cell, an electric potential is created between two dissimilar metals. Detailed explanation of standard electrode potential provided here. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she),. Standard Potential Electrode Reaction.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. Describe and relate the definitions of electrode and cell potentials. In an electrochemical cell, an electric potential is created between two dissimilar metals. Since the potential of the s.h.e. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode potential. Standard Potential Electrode Reaction.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. In an electrochemical cell, an electric potential is created between two dissimilar metals. Interpret electrode potentials in terms of relative oxidant and reductant. Since the potential of the s.h.e. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode potential. Standard Potential Electrode Reaction.
From www.aakash.ac.in
Standard Electrode Potential Importance, Definition & Redox Reactions Standard Potential Electrode Reaction Standard electrode potential is the potential that arises in a cell under standard conditions. In an electrochemical cell, an electric potential is created between two dissimilar metals. Interpret electrode potentials in terms of relative oxidant and reductant. Since the potential of the s.h.e. Detailed explanation of standard electrode potential provided here. Describe and relate the definitions of electrode and cell. Standard Potential Electrode Reaction.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Potential Electrode Reaction Standard electrode potential is the potential that arises in a cell under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Detailed explanation of standard electrode potential. Standard Potential Electrode Reaction.
From chem.libretexts.org
6.2 Standard Electrode Potentials Chemistry LibreTexts Standard Potential Electrode Reaction Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Detailed explanation of standard electrode potential provided here. Standard electrode potential is the potential that arises in a cell under standard conditions. Describe and relate the definitions of electrode and. Standard Potential Electrode Reaction.
From question.pandai.org
Standard Electrode Potential Standard Potential Electrode Reaction Interpret electrode potentials in terms of relative oxidant and reductant. Since the potential of the s.h.e. Standard electrode potential is the potential that arises in a cell under standard conditions. Detailed explanation of standard electrode potential provided here. In an electrochemical cell, an electric potential is created between two dissimilar metals. 372 rows the data below tabulates standard electrode potentials. Standard Potential Electrode Reaction.
From testbook.com
Standard Electrode PotentialLearn Definition,Formula,Conditions Standard Potential Electrode Reaction Detailed explanation of standard electrode potential provided here. Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode potential is the potential that arises in a cell under standard conditions. In an electrochemical cell, an electric potential is. Standard Potential Electrode Reaction.
From 2012books.lardbucket.org
Standard Potentials Standard Potential Electrode Reaction 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In an electrochemical cell, an electric potential is created between two dissimilar metals. Describe and relate the definitions of electrode and cell potentials. Since the potential of the s.h.e. Standard electrode potential is the potential that arises in a cell. Standard Potential Electrode Reaction.
From users.highland.edu
Standard Potentials Standard Potential Electrode Reaction Standard electrode potential is the potential that arises in a cell under standard conditions. Since the potential of the s.h.e. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Detailed explanation of standard electrode potential provided here. In an electrochemical cell, an electric potential is created between two dissimilar. Standard Potential Electrode Reaction.
From notes.pegas.is
Types of Reactions Pega's Notes Standard Potential Electrode Reaction Describe and relate the definitions of electrode and cell potentials. Standard electrode potential is the potential that arises in a cell under standard conditions. Since the potential of the s.h.e. In an electrochemical cell, an electric potential is created between two dissimilar metals. Interpret electrode potentials in terms of relative oxidant and reductant. Detailed explanation of standard electrode potential provided. Standard Potential Electrode Reaction.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Redox (II) Standard Electrode Potential Eo (1) Standard Potential Electrode Reaction Describe and relate the definitions of electrode and cell potentials. In an electrochemical cell, an electric potential is created between two dissimilar metals. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Standard electrode potential is the potential that arises in a cell under standard conditions. Detailed explanation of. Standard Potential Electrode Reaction.