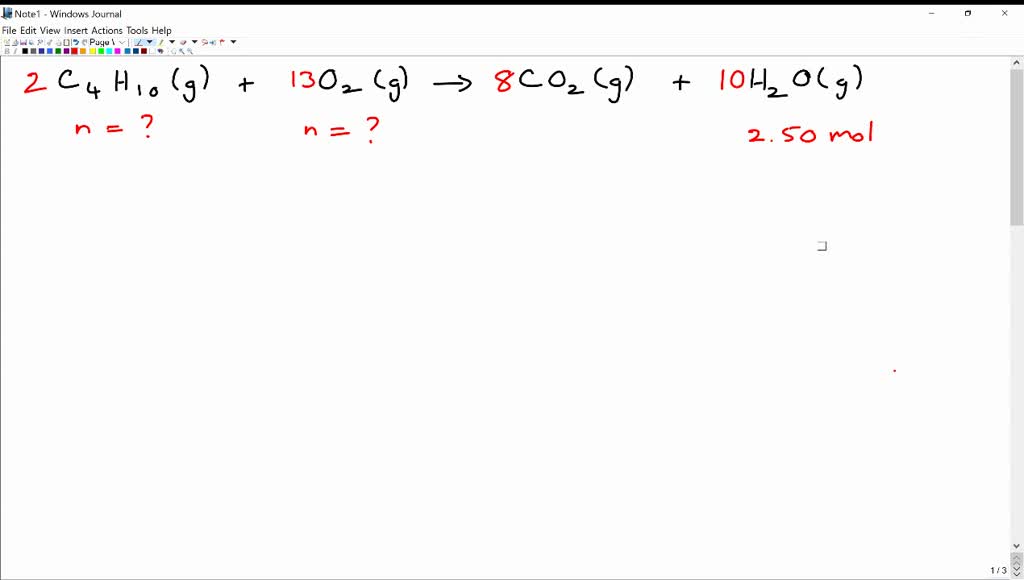Butane Equation Balanced . Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). Balance any equation or reaction using this chemical equation balancer! To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the gaseous phase: Find out what type of reaction occured. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. The chemical formula of butane is #c_4h_10#. The combustion of butane is a reaction between butane and oxygen gas that. C4h10 +o2 → 4co2 +. With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down.
from www.numerade.com
The chemical formula of butane is #c_4h_10#. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Find out what type of reaction occured. C4h10 +o2 → 4co2 +. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. Balance any equation or reaction using this chemical equation balancer! Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. The combustion of butane is a reaction between butane and oxygen gas that. To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you.
SOLVEDThe balanced chemical equation for the reaction between butane (C4H1o) and oxygen is
Butane Equation Balanced With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the gaseous phase: Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. The chemical formula of butane is #c_4h_10#. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. C4h10 +o2 → 4co2 +. The combustion of butane is a reaction between butane and oxygen gas that.
From www.chegg.com
Solved The balanced equation for the combustion of butane Butane Equation Balanced To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The combustion of butane is a reaction between butane and oxygen gas that. Balance any equation or reaction using this chemical equation. Butane Equation Balanced.
From www.numerade.com
SOLVED Write balanced equation for the combustion of gaseous butane (C4H10) fuel used in Butane Equation Balanced Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the. Butane Equation Balanced.
From www.coursehero.com
[Solved] The balanced equation for the combustion of butane is 2CH10+ 1302... Course Hero Butane Equation Balanced Find out what type of reaction occured. Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. Balance. Butane Equation Balanced.
From brainly.com
Based on the information, what is the balanced chemical equation representing the burning of Butane Equation Balanced The combustion of butane is a reaction between butane and oxygen gas that. Balance any equation or reaction using this chemical equation balancer! Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write. Butane Equation Balanced.
From www.coursehero.com
[Solved] 6.. The balanced equation for the complete combustion of butane is... Course Hero Butane Equation Balanced C4h10 +o2 → 4co2 +. Find out what type of reaction occured. The chemical formula of butane is #c_4h_10#. To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon. Butane Equation Balanced.
From treatybottle13.pythonanywhere.com
Marvelous Balanced Equation For Butane And Oxygen Formula Photosynthesis Butane Equation Balanced Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. C4h10 +o2 → 4co2 +. The chemical formula of butane is #c_4h_10#. Balance any equation or reaction. Butane Equation Balanced.
From www.numerade.com
SOLVED The balanced equation for the combustion of butane is 2C4H10(g) + 13O2(g) → 8CO2(g Butane Equation Balanced Find out what type of reaction occured. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. The combustion of butane is a reaction between butane and oxygen gas that. With butane. Butane Equation Balanced.
From www.chegg.com
Solved Determine the balanced chemical equation for butane Butane Equation Balanced The combustion of butane is a reaction between butane and oxygen gas that. C4h10 +o2 → 4co2 +. Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). The chemical formula of butane is #c_4h_10#. With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. C4h10. Butane Equation Balanced.
From www.numerade.com
SOLVED The balanced equation for the combustion of butane, C4H10, is 2 C4H10(g) + 13 O2(g) → 8 Butane Equation Balanced To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. The chemical formula of butane is #c_4h_10#. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. C4h10 +o2 → 4co2 +. Find. Butane Equation Balanced.
From www.chegg.com
Solved The balanced equation for the combustion of butane Butane Equation Balanced To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Butane (c4h10). Butane Equation Balanced.
From www.youtube.com
Balance combustion Butane 20pct excess 85pct consumed YouTube Butane Equation Balanced Balance any equation or reaction using this chemical equation balancer! The chemical formula of butane is #c_4h_10#. With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. Find out what type of reaction occured. Limiting reagent can be computed for a balanced equation by entering the number of moles or. Butane Equation Balanced.
From www.numerade.com
SOLVED The balanced equation for the combustion of butane is 2C4H10+13O28CO2+10H2O If the Butane Equation Balanced C4h10 +o2 → 4co2 +. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Balance any equation or reaction using this chemical equation balancer! To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. C4h10 + o2 = co + h2o. Butane Equation Balanced.
From www.numerade.com
SOLVED Butane, C4H10, is a component of natural gas that is used as fuel for cigarette lighters Butane Equation Balanced Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). The chemical formula of butane is #c_4h_10#. With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! The combustion. Butane Equation Balanced.
From www.numerade.com
SOLVED write a balanced equation showing the reaction of butane with oxygen gas to form carbon Butane Equation Balanced Balance any equation or reaction using this chemical equation balancer! C4h10 +o2 → 4co2 +. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The combustion of butane is a reaction between butane and oxygen gas that. To balance the chemical equation for the combustion of butane (c4h10 +. Butane Equation Balanced.
From www.numerade.com
The balanced equation for the combustion of butane is 2C4H10 + 13O2 —> 8CO2 + 10H2O. 1st Butane Equation Balanced Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the gaseous phase: C4h10 +o2 → 4co2 +. Find out what type of reaction occured. The combustion of butane is a reaction between butane and oxygen gas that. Balance any equation or reaction. Butane Equation Balanced.
From www.numerade.com
SOLVED 1. Butane is typically used as fuel for cigarette lighters and portable stoves. What Butane Equation Balanced Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The combustion of butane is a reaction between butane. Butane Equation Balanced.
From www.coursehero.com
[Solved] Using the balanced equation for the combustion of butane (C4H,( ),... Course Hero Butane Equation Balanced Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the gaseous phase: C4h10 +o2 → 4co2 +. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles. Butane Equation Balanced.
From www.chegg.com
Solved The balanced chemical equation for the combustion of Butane Equation Balanced C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. The combustion of butane is a reaction between butane and oxygen gas that.. Butane Equation Balanced.
From treatybottle13.pythonanywhere.com
Marvelous Balanced Equation For Butane And Oxygen Formula Photosynthesis Butane Equation Balanced C4h10 +o2 → 4co2 +. The combustion of butane is a reaction between butane and oxygen gas that. Find out what type of reaction occured. Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. C4h10. Butane Equation Balanced.
From www.numerade.com
SOLVED Butane, C4H10, is a component of natural gas that is used as fuel for cigarette lighters Butane Equation Balanced Find out what type of reaction occured. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The chemical formula of butane is #c_4h_10#. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of. Butane Equation Balanced.
From www.numerade.com
SOLVED Write the balanced chemical equation for the complete combustion of butane (g) . Express Butane Equation Balanced Find out what type of reaction occured. To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the gaseous phase: Balance any equation or reaction. Butane Equation Balanced.
From www.coursehero.com
[Solved] The balanced equation for the combustion of butane is 2CH10+ 1302... Course Hero Butane Equation Balanced Balance any equation or reaction using this chemical equation balancer! With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. C4h10 +o2 → 4co2 +. Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). To balance the chemical equation for the combustion of butane (c4h10. Butane Equation Balanced.
From www.coursehero.com
[Solved] The balanced equation for the combustion of butane is 2CH10+ 1302... Course Hero Butane Equation Balanced Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). The chemical formula of butane is #c_4h_10#. Balance any equation or reaction using this chemical equation balancer! Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the gaseous. Butane Equation Balanced.
From www.alamy.com
The illustration of the butane structural formula Stock Vector Image & Art Alamy Butane Equation Balanced Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Balance any equation or reaction using this chemical equation balancer! Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 +. Butane Equation Balanced.
From www.numerade.com
SOLVEDUse the balanced equation for the combustion of butane to complete the table. Butane Equation Balanced C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. The combustion of butane is a reaction between butane and oxygen gas that. Balance any equation or reaction using this chemical equation balancer! With butane (c 4 h 10), you can. Butane Equation Balanced.
From www.coursehero.com
[Solved] Using the balanced equation for the combustion of butane (C4H,( ),... Course Hero Butane Equation Balanced The combustion of butane is a reaction between butane and oxygen gas that. Find out what type of reaction occured. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4. Butane Equation Balanced.
From www.coursehero.com
[Solved] 6.. The balanced equation for the complete combustion of butane is... Course Hero Butane Equation Balanced With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. Balance any equation or reaction using this chemical equation balancer! Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the gaseous phase:. Butane Equation Balanced.
From www.numerade.com
SOLVED Consider the following balanced equation for the combustion of butane, a fuel often used Butane Equation Balanced Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the gaseous phase: Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide. Butane Equation Balanced.
From www.numerade.com
Consider the following balanced equation for the combustion of butane, a fuel often used in Butane Equation Balanced Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). The combustion of butane is a reaction between butane and oxygen gas that. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. The chemical formula of. Butane Equation Balanced.
From byjus.com
Write the structural formula for butane. Butane Equation Balanced C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Balance any equation or reaction using this chemical equation balancer! The. Butane Equation Balanced.
From www.chegg.com
The balanced equation for the combustion of butane Butane Equation Balanced The combustion of butane is a reaction between butane and oxygen gas that. Find out what type of reaction occured. Butane (c4h10) reacts with oxygen (o2) to make carbon dioxide (co2) and water (h2o). Balance any equation or reaction using this chemical equation balancer! C4h10 +o2 → 4co2 +. C4h10 + o2 = co + h2o is a double displacement. Butane Equation Balanced.
From www.vectorstock.com
Butane formula Royalty Free Vector Image VectorStock Butane Equation Balanced C4h10 +o2 → 4co2 +. Balance the chemical equation for the combustion of butane liquid (c 4 h 10) in the presence of oxygen gas to yield carbon dioxide and water in the gaseous phase: Balance any equation or reaction using this chemical equation balancer! With butane (c 4 h 10), you can again balance the carbons and hydrogens as. Butane Equation Balanced.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between butane (C4H10) and oxygen is 2 Butane Equation Balanced C4h10 +o2 → 4co2 +. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10] and nine moles of dioxygen [o 2]. The combustion of butane is a reaction between butane and oxygen gas that. Balance the chemical equation for the combustion of butane liquid (c 4 h. Butane Equation Balanced.
From www.numerade.com
SOLVEDThe balanced chemical equation for the reaction between butane (C4H1o) and oxygen is Butane Equation Balanced With butane (c 4 h 10), you can again balance the carbons and hydrogens as you write the equation down. To balance the chemical equation for the combustion of butane (c4h10 + o2 = co2 + h2o) you. C4h10 + o2 = co + h2o is a double displacement (metathesis) reaction where two moles of butane [c 4 h 10]. Butane Equation Balanced.
From www.numerade.com
SOLVED (5 pts) The balanced equation for the combustion of butane is 2C4H10(g) + 13 O2(g) → 8 Butane Equation Balanced Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The chemical formula of butane is #c_4h_10#. With butane (c 4 h 10), you can again balance the carbons and hydrogens as you. Butane Equation Balanced.