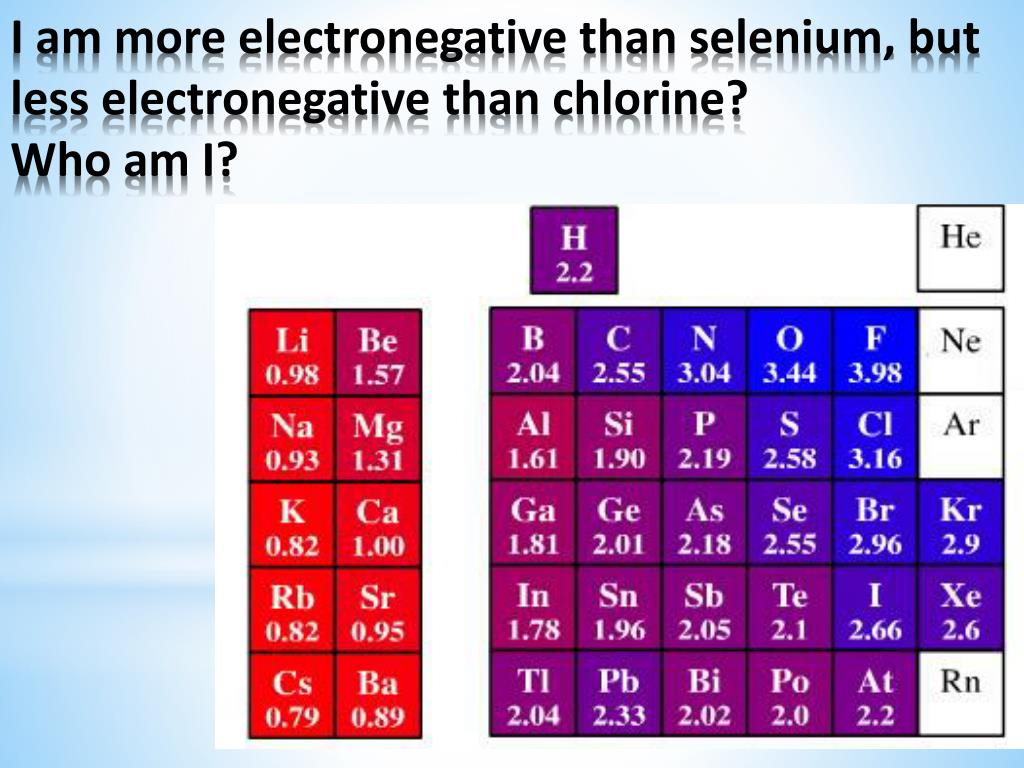
Chlorine Has Greater Electronegativity . 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It can also be used to predict if. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Why does electronegativity increase across a period? Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Among the elements, it has the highest. 3.16 is the electronegativity value of chlorine (cl). It is an extremely reactive element and a strong oxidising agent: The suggested values are all taken from. Why does electronegativity increase across a period? It is among the highly reactive non. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens.
from www.slideserve.com
It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Why does electronegativity increase across a period? It can also be used to predict if. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Why does electronegativity increase across a period? Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. It is an extremely reactive element and a strong oxidising agent: 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The suggested values are all taken from.
PPT Properties of Water PowerPoint Presentation, free download ID3229568
Chlorine Has Greater Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It can also be used to predict if. The suggested values are all taken from. 3.16 is the electronegativity value of chlorine (cl). Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It is an extremely reactive element and a strong oxidising agent: Why does electronegativity increase across a period? Why does electronegativity increase across a period? 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It is among the highly reactive non. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Among the elements, it has the highest.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine is 3.6ev/ atom. The Chlorine Has Greater Electronegativity 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. It is among the highly reactive non. Consider sodium at the beginning of period 3 and. Chlorine Has Greater Electronegativity.
From www.coursehero.com
[Solved] . 1. Chlorine has a higher electronegativity than sulfur. T / F... Course Hero Chlorine Has Greater Electronegativity It is an extremely reactive element and a strong oxidising agent: Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. 103 rows electronegativity is. Chlorine Has Greater Electronegativity.
From www.numerade.com
SOLVED In hydrochloric acid (HCI , chlorine has greater electronegativity than hydrogen Chlorine Has Greater Electronegativity Why does electronegativity increase across a period? Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Why does electronegativity increase across a period? It is among the highly reactive non. It can also be used to predict if. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons. Chlorine Has Greater Electronegativity.
From therealgroupichem.weebly.com
Electronegativity Group i Chemistry(iiiescuro) Chlorine Has Greater Electronegativity It can also be used to predict if. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Among the elements, it has the highest. 3.16 is the electronegativity value of chlorine (cl). The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the. Chlorine Has Greater Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine Has Greater Electronegativity Among the elements, it has the highest. It can also be used to predict if. It is among the highly reactive non. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Why does electronegativity increase across. Chlorine Has Greater Electronegativity.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Has Greater Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Why does electronegativity increase across a period? 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all taken from. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons. Chlorine Has Greater Electronegativity.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Has Greater Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the. Chlorine Has Greater Electronegativity.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Chlorine Has Greater Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Why does electronegativity increase across a period? 3.16 is the electronegativity value of chlorine (cl). It is an extremely reactive element and a strong oxidising agent: Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 103. Chlorine Has Greater Electronegativity.
From www.wikihow.com
4 Ways to Calculate Electronegativity wikiHow Chlorine Has Greater Electronegativity Among the elements, it has the highest. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. 3.16 is the electronegativity value of chlorine. Chlorine Has Greater Electronegativity.
From chemistry.com.pk
electronegativity chart Chlorine Has Greater Electronegativity The suggested values are all taken from. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The chlorine atom. Chlorine Has Greater Electronegativity.
From www.periodic-table.org
Chlorine Electronegativity Cl Chlorine Has Greater Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Why does electronegativity increase across a period? 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 3.16 is the electronegativity value of chlorine (cl). Why does electronegativity increase across a period? Among the elements, it has the highest.. Chlorine Has Greater Electronegativity.
From www.youtube.com
Which has a greater electronegativity value Na or Cl ? YouTube Chlorine Has Greater Electronegativity 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It is an extremely reactive element and a strong oxidising agent: 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if. It is among the highly reactive non. It belongs. Chlorine Has Greater Electronegativity.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Has Greater Electronegativity It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if. It is. Chlorine Has Greater Electronegativity.
From secrets-of-periodic-table.blogspot.com
PERIODIC TABLE ELECTRONEGATIVITY NOBLE GASES PAULING ELECTRONEGATIVITY VALUES Chlorine Has Greater Electronegativity It is among the highly reactive non. The suggested values are all taken from. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Among the elements, it has the highest. It belongs to the 7th group and 2nd period on. Chlorine Has Greater Electronegativity.
From periodictable.me
How to Calculate Electronegativity of Element Chlorine Has Greater Electronegativity Among the elements, it has the highest. Why does electronegativity increase across a period? It is among the highly reactive non. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 3.16 is the electronegativity value of chlorine. Chlorine Has Greater Electronegativity.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure and electron configuration Chlorine Has Greater Electronegativity 3.16 is the electronegativity value of chlorine (cl). 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Why does electronegativity increase across a period? Why does electronegativity increase across a period? Among the elements, it has the highest. It belongs to the 7th group and 2nd period on the periodic table, known as the. Chlorine Has Greater Electronegativity.
From slideplayer.com
Hydrogen and Chlorine ppt download Chlorine Has Greater Electronegativity Among the elements, it has the highest. It is an extremely reactive element and a strong oxidising agent: The suggested values are all taken from. 3.16 is the electronegativity value of chlorine (cl). The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the. Chlorine Has Greater Electronegativity.
From www.dreamstime.com
Chlorine Chemical Element with 17 Atomic Number, Atomic Mass and Electronegativity Values Chlorine Has Greater Electronegativity The suggested values are all taken from. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. It is among the highly reactive non. Why does electronegativity increase across a period? 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It can also be used to predict if.. Chlorine Has Greater Electronegativity.
From www.numerade.com
Which of the following statements is true regarding sodium and chlorine? (A) Sodium has greater Chlorine Has Greater Electronegativity 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 3.16 is the electronegativity value of chlorine (cl). The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Why does electronegativity increase across a. Chlorine Has Greater Electronegativity.
From socratic.org
Which group of elements is listed in order of increasing electronegativity? a) F, Cl, Ge, Sn b Chlorine Has Greater Electronegativity The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. The suggested values are all taken from. It belongs to the 7th group and 2nd. Chlorine Has Greater Electronegativity.
From www.aiophotoz.com
Periodic Table Of Elements Electronegativity Chart Periodic Table Images and Photos finder Chlorine Has Greater Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Among the elements, it has the highest. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. The chlorine atom has a higher. Chlorine Has Greater Electronegativity.
From www.chegg.com
Solved Hydrochloric acid (HCl) is a molecule. Chlorine has Chlorine Has Greater Electronegativity Why does electronegativity increase across a period? 103 rows electronegativity is not a uniquely defined property and may depend on the definition. It can also be used to predict if. It is an extremely reactive element and a strong oxidising agent: 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the. Chlorine Has Greater Electronegativity.
From www.numerade.com
Using the periodic table, which element in each pair is more electronegative? (a) Si, O (b) N, C Chlorine Has Greater Electronegativity Why does electronegativity increase across a period? It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The suggested values are all taken from. 3.16 is the electronegativity value of chlorine (cl). 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Consider sodium at the beginning of. Chlorine Has Greater Electronegativity.
From www.slideserve.com
PPT Electronegativity PowerPoint Presentation, free download ID256407 Chlorine Has Greater Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. It can also be used to predict if. Among the elements, it has the highest. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all taken from. It is among the highly reactive non.. Chlorine Has Greater Electronegativity.
From www.youtube.com
Why electronegativity of chlorine is greater than of fluorine? Periodic TableNcert YouTube Chlorine Has Greater Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. It is an extremely reactive element and a strong oxidising agent: The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Consider sodium at the. Chlorine Has Greater Electronegativity.
From iperiodictable.com
Periodic Table Electronegativity Periodic Table Chlorine Has Greater Electronegativity Why does electronegativity increase across a period? It can also be used to predict if. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. It is an extremely reactive element and a strong oxidising agent: Consider sodium at. Chlorine Has Greater Electronegativity.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Has Greater Electronegativity It is an extremely reactive element and a strong oxidising agent: The suggested values are all taken from. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in. Chlorine Has Greater Electronegativity.
From mavink.com
Electronegativity Difference Chart Chlorine Has Greater Electronegativity It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It is among the highly reactive non. It can also be used to predict if. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Why does electronegativity increase across a period? 3.16 is the electronegativity. Chlorine Has Greater Electronegativity.
From www.numerade.com
SOLVED Sodium (Na) and hydrogen (H) have the same number of electrons in their outer shells but Chlorine Has Greater Electronegativity It is among the highly reactive non. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 103 rows electronegativity is. Chlorine Has Greater Electronegativity.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID3229568 Chlorine Has Greater Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the. Chlorine Has Greater Electronegativity.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity Values Chlorine Has Greater Electronegativity Why does electronegativity increase across a period? Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. The suggested values are all taken from. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It can also be used. Chlorine Has Greater Electronegativity.
From www.youtube.com
Electronegativity, Basic Introduction, Periodic Trends Which Element Is More Electronegative Chlorine Has Greater Electronegativity The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Why does electronegativity increase across a period? Why does electronegativity increase across a period? It belongs to the 7th group and 2nd period on the periodic table, known as the halogens.. Chlorine Has Greater Electronegativity.
From mungfali.com
Electronegativity Trend Periodic Table Chlorine Has Greater Electronegativity It is among the highly reactive non. It can also be used to predict if. It is an extremely reactive element and a strong oxidising agent: Why does electronegativity increase across a period? 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Among the elements, it has the highest. Why does electronegativity. Chlorine Has Greater Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine Has Greater Electronegativity Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Why does electronegativity increase across a period? 3.16 is the electronegativity value of chlorine (cl). 103 rows electronegativity is not a uniquely defined property and may depend. Chlorine Has Greater Electronegativity.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Chlorine Has Greater Electronegativity Why does electronegativity increase across a period? It can also be used to predict if. The suggested values are all taken from. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows electronegativity is not a uniquely defined property and may depend on the definition. 3.16 is the electronegativity value of. Chlorine Has Greater Electronegativity.