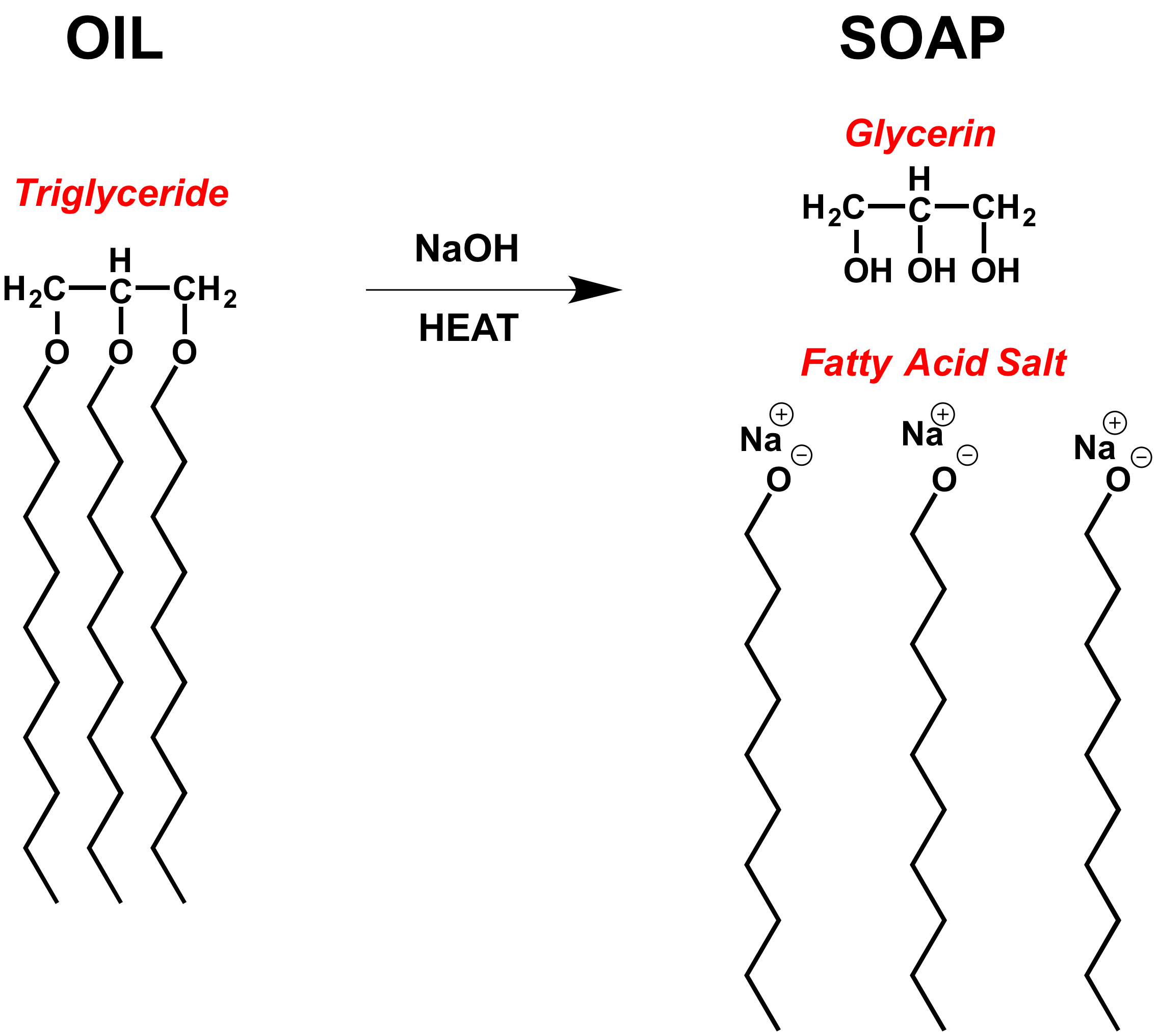
Soap Chemical Bond . C=c bonds cause the fatty. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. Soap is a classic cleaning agent that has been used for centuries. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. soaps act as cleansers because the two ends of a soap molecule are so different. Its fundamental chemistry involves the combination. This combination creates clusters of soap, water, and grime called micelles. Unsaturated fatty acids contain double and/or triple bonds.
from bossanovasoap.com
Soap is a classic cleaning agent that has been used for centuries. C=c bonds cause the fatty. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. This combination creates clusters of soap, water, and grime called micelles. Unsaturated fatty acids contain double and/or triple bonds. Its fundamental chemistry involves the combination. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. soaps act as cleansers because the two ends of a soap molecule are so different.
CHEMISTRY
Soap Chemical Bond This combination creates clusters of soap, water, and grime called micelles. C=c bonds cause the fatty. This combination creates clusters of soap, water, and grime called micelles. Its fundamental chemistry involves the combination. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. soaps act as cleansers because the two ends of a soap molecule are so different. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Soap is a classic cleaning agent that has been used for centuries. Unsaturated fatty acids contain double and/or triple bonds.
From www.thoughtco.com
How Soap Works Soap Chemical Bond Its fundamental chemistry involves the combination. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. This combination creates clusters of soap, water, and grime called micelles. Unsaturated fatty acids contain double and/or triple bonds. Soap is a classic cleaning agent that has been used for centuries. C=c bonds. Soap Chemical Bond.
From www.reddit.com
Chemistry of Soap versus Body Wash r/chemistry Soap Chemical Bond if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Unsaturated fatty acids contain double and/or triple bonds. This combination creates clusters of soap, water, and grime called micelles. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that. Soap Chemical Bond.
From www.scribd.com
Soaps and Detergents Cleansing Action of Soap PDF Chemical Bond Soap Chemical Bond C=c bonds cause the fatty. soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a classic cleaning agent that has been used for centuries. Its fundamental chemistry involves the combination. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap. Soap Chemical Bond.
From www.youtube.com
Chemistry 101 How does soap work? YouTube Soap Chemical Bond soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. Unsaturated fatty acids contain double and/or triple bonds. Its fundamental chemistry involves the combination. C=c bonds cause the fatty. soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a classic. Soap Chemical Bond.
From www.slideserve.com
PPT Soap Describe how soap is made from fatty acids and alkalis Soap Chemical Bond This combination creates clusters of soap, water, and grime called micelles. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. soaps act as cleansers because the two ends of a soap molecule are so different. Its fundamental. Soap Chemical Bond.
From www.youtube.com
How Does Soap affect Hydrogen Bonds YouTube Soap Chemical Bond Soap is a classic cleaning agent that has been used for centuries. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. C=c bonds cause the fatty. Its fundamental chemistry involves the combination. soaps act as cleansers because the two ends of a soap molecule are so different.. Soap Chemical Bond.
From ar.inspiredpencil.com
Soap Molecule Polar Or Nonpolar Soap Chemical Bond C=c bonds cause the fatty. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a classic cleaning agent that has. Soap Chemical Bond.
From www.youtube.com
How Soap Works and Chemical Bonds YouTube Soap Chemical Bond Unsaturated fatty acids contain double and/or triple bonds. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. how soap works is due to its unique chemistry,. Soap Chemical Bond.
From cosmosmagazine.com
The chemistry of soap Soap Chemical Bond soaps act as cleansers because the two ends of a soap molecule are so different. Its fundamental chemistry involves the combination. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. Soap is a classic cleaning agent that has been used for centuries. This combination creates clusters of. Soap Chemical Bond.
From spmchemistry.blog.onlinetuition.com.my
Detergent SPM Chemistry Soap Chemical Bond soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a classic cleaning agent that has been used for centuries. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. Unsaturated fatty acids contain double and/or triple bonds. if the. Soap Chemical Bond.
From www.slideserve.com
PPT Making Soap PowerPoint Presentation, free download ID1971971 Soap Chemical Bond Soap is a classic cleaning agent that has been used for centuries. Unsaturated fatty acids contain double and/or triple bonds. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. soaps act as cleansers because the two ends. Soap Chemical Bond.
From www.pinterest.com
How Soap Work? Soap, Cleanse, Basic concepts Soap Chemical Bond Its fundamental chemistry involves the combination. Soap is a classic cleaning agent that has been used for centuries. C=c bonds cause the fatty. soaps act as cleansers because the two ends of a soap molecule are so different. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap. Soap Chemical Bond.
From www.sharpestarena.com
Production of Soap Complete Project on Soap Making Soap Chemical Bond how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. C=c bonds cause the fatty. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Its fundamental chemistry. Soap Chemical Bond.
From www.dreamstime.com
General Formula of Solid and Liquid Soap Molecule. RCOONa, RCOOK Soap Chemical Bond Unsaturated fatty acids contain double and/or triple bonds. This combination creates clusters of soap, water, and grime called micelles. Its fundamental chemistry involves the combination. soaps act as cleansers because the two ends of a soap molecule are so different. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts. Soap Chemical Bond.
From ffden-2.phys.uaf.edu
Soap and Water Interaction Soap Chemical Bond Unsaturated fatty acids contain double and/or triple bonds. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids.. Soap Chemical Bond.
From bossanovasoap.com
CHEMISTRY Soap Chemical Bond C=c bonds cause the fatty. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. soaps act. Soap Chemical Bond.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID Soap Chemical Bond C=c bonds cause the fatty. Soap is a classic cleaning agent that has been used for centuries. Its fundamental chemistry involves the combination. Unsaturated fatty acids contain double and/or triple bonds. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. how soap works is due to its. Soap Chemical Bond.
From www.thoughtco.com
How Saponification Makes Soap Soap Chemical Bond C=c bonds cause the fatty. Soap is a classic cleaning agent that has been used for centuries. Unsaturated fatty acids contain double and/or triple bonds. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. soaps act as. Soap Chemical Bond.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences Soap Chemical Bond Unsaturated fatty acids contain double and/or triple bonds. This combination creates clusters of soap, water, and grime called micelles. Soap is a classic cleaning agent that has been used for centuries. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. C=c bonds cause the fatty. soaps act. Soap Chemical Bond.
From www.alamy.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK Soap Chemical Bond how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. soaps act as cleansers because the two ends of a soap molecule are so different. C=c bonds cause the fatty. This combination creates clusters of soap, water, and. Soap Chemical Bond.
From cbse.myindialist.com
Chemistry X Carbon and its Compounds SOAPS AND DETERGENTS CBSE Soap Chemical Bond if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Unsaturated fatty acids contain double and/or triple bonds. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil.. Soap Chemical Bond.
From ar.inspiredpencil.com
Soap Molecule Structure Soap Chemical Bond Unsaturated fatty acids contain double and/or triple bonds. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. C=c bonds cause the fatty. soaps act as cleansers because the two ends of a soap molecule are so different.. Soap Chemical Bond.
From www.shutterstock.com
Soap Chemistry Soap Chemistry Formula Structure Stock Vector (Royalty Soap Chemical Bond how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Its fundamental chemistry involves the combination. Unsaturated fatty. Soap Chemical Bond.
From app.emaze.com
Presentation Name on emaze Soap Chemical Bond Unsaturated fatty acids contain double and/or triple bonds. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. how soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil.. Soap Chemical Bond.
From courses.lumenlearning.com
17.2 Fats and Oils The Basics of General, Organic, and Biological Soap Chemical Bond soaps act as cleansers because the two ends of a soap molecule are so different. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. C=c bonds. Soap Chemical Bond.
From www.coursehero.com
[Solved] Draw the chemical structure of a soap and a detergent (choose Soap Chemical Bond if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. soaps act as cleansers because the two ends of a soap molecule are so different. C=c bonds cause the fatty. Unsaturated fatty acids contain double and/or triple bonds. soap molecules have a hybrid structure, with a hydrophilic. Soap Chemical Bond.
From www.scribd.com
Soap Soap Chemical Substances Soap Chemical Bond Its fundamental chemistry involves the combination. C=c bonds cause the fatty. Unsaturated fatty acids contain double and/or triple bonds. This combination creates clusters of soap, water, and grime called micelles. soaps act as cleansers because the two ends of a soap molecule are so different. soap molecules have a hybrid structure, with a hydrophilic head that bonds to. Soap Chemical Bond.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soap Chemical Bond soaps act as cleansers because the two ends of a soap molecule are so different. Unsaturated fatty acids contain double and/or triple bonds. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. how soap works is due to its unique chemistry, the hydrophilic (loves water) and. Soap Chemical Bond.
From exoypyvpw.blob.core.windows.net
How To Prepare Soap In The Laboratory at Arthur Lagasse blog Soap Chemical Bond Unsaturated fatty acids contain double and/or triple bonds. C=c bonds cause the fatty. This combination creates clusters of soap, water, and grime called micelles. Its fundamental chemistry involves the combination. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. soaps act as cleansers because the two ends. Soap Chemical Bond.
From stock.adobe.com
Saponification equation, reaction of soap, chemistry equation of soap Soap Chemical Bond soaps act as cleansers because the two ends of a soap molecule are so different. soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. Unsaturated fatty acids contain double and/or triple bonds. C=c bonds cause the fatty. Its fundamental chemistry involves the combination. This combination creates clusters. Soap Chemical Bond.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soap Chemical Bond This combination creates clusters of soap, water, and grime called micelles. Soap is a classic cleaning agent that has been used for centuries. soaps act as cleansers because the two ends of a soap molecule are so different. Unsaturated fatty acids contain double and/or triple bonds. soap molecules have a hybrid structure, with a hydrophilic head that bonds. Soap Chemical Bond.
From www.pinterest.com
Cleansing Action Of Soap. Soap, Cleanse, Molecules Soap Chemical Bond soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. Soap is a classic cleaning agent that has been used for centuries. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Its fundamental chemistry involves the combination.. Soap Chemical Bond.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Soap Chemical Bond soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. soaps act as cleansers because the two ends of a soap molecule are so different. Unsaturated fatty acids contain double and/or triple bonds. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty. Soap Chemical Bond.
From exoxmnoxz.blob.core.windows.net
How Does Soap Work Science at Kelly Kline blog Soap Chemical Bond soap molecules have a hybrid structure, with a hydrophilic head that bonds to water and a hydrophobic tail that avoids. soaps act as cleansers because the two ends of a soap molecule are so different. Soap is a classic cleaning agent that has been used for centuries. C=c bonds cause the fatty. how soap works is due. Soap Chemical Bond.
From www.slideshare.net
Soap and detergents Soap Chemical Bond C=c bonds cause the fatty. This combination creates clusters of soap, water, and grime called micelles. if the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. Soap is a classic cleaning agent that has been used for centuries. Its fundamental chemistry involves the combination. Unsaturated fatty acids contain double. Soap Chemical Bond.