Standard Heat Of Formation Potassium Hydroxide . Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k:
from www.chegg.com
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies.
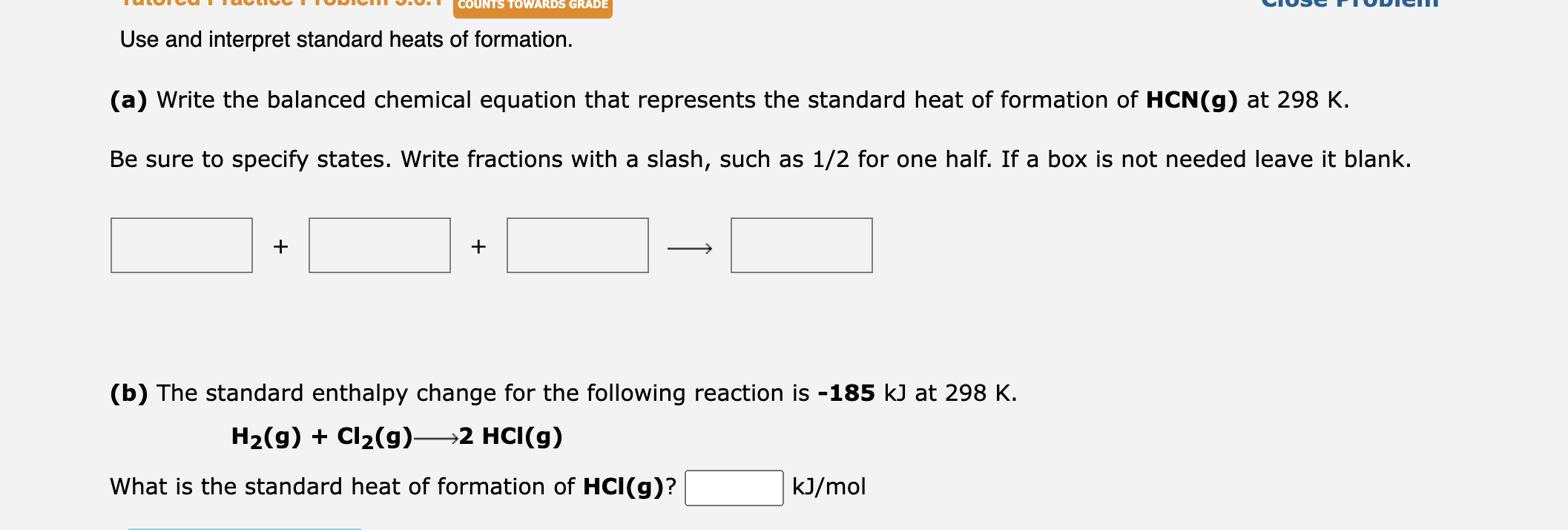
Solved Use and interpret standard heats of formation. (a)
Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies.
From learningschoolandy.z21.web.core.windows.net
Heat Of Formation List Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h 136 rows standard enthalpy change of formation (data table) these tables include heat of. Standard Heat Of Formation Potassium Hydroxide.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Potassium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t. Standard Heat Of Formation Potassium Hydroxide.
From slideplayer.com
Chapter 17 “Thermochemistry” ppt download Standard Heat Of Formation Potassium Hydroxide Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f −. Standard Heat Of Formation Potassium Hydroxide.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond. Standard Heat Of Formation Potassium Hydroxide.
From www.numerade.com
SOLVED a) Calculate the standard heat of the neutralization reaction Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Heat Of Formation Potassium Hydroxide.
From www.chegg.com
Solved (a) Write the balanced chemical equation that Standard Heat Of Formation Potassium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 +. Standard Heat Of Formation Potassium Hydroxide.
From www.chegg.com
Solved Select the correct reaction for the standard heat of Standard Heat Of Formation Potassium Hydroxide 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Heat Of Formation Potassium Hydroxide.
From www.researchgate.net
14. Specific heat of potassium formatewater Download Scientific Diagram Standard Heat Of Formation Potassium Hydroxide Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Gas. Standard Heat Of Formation Potassium Hydroxide.
From classdbmarshall.z13.web.core.windows.net
Heat Of Formation Formula Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature =. Standard Heat Of Formation Potassium Hydroxide.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Heat Of Formation Potassium Hydroxide Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. 136 rows standard enthalpy change of formation (data table). Standard Heat Of Formation Potassium Hydroxide.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Potassium Hydroxide Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f −. Standard Heat Of Formation Potassium Hydroxide.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h 136 rows standard enthalpy change of formation (data table) these tables include heat of. Standard Heat Of Formation Potassium Hydroxide.
From www.studocu.com
The Heat of Reaction of Potassium Hydroxide and Hydrochloric Acid 2 Standard Heat Of Formation Potassium Hydroxide 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. Potassium hydroxide (koh) h 1 k. Standard Heat Of Formation Potassium Hydroxide.
From www.chegg.com
Solved 10. At 298 K, the standard heat of formation, A_H", Standard Heat Of Formation Potassium Hydroxide Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Heat Of Formation Potassium Hydroxide.
From slideplayer.com
Heat in Changes of State and Calculating Heat of Reaction ppt download Standard Heat Of Formation Potassium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety. Standard Heat Of Formation Potassium Hydroxide.
From www.chegg.com
Solved D Hess's Law Calculations Use the standard heats of Standard Heat Of Formation Potassium Hydroxide 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: 193 rows in chemistry. Standard Heat Of Formation Potassium Hydroxide.
From www.bartleby.com
Answered Write a balanced chemical equation… bartleby Standard Heat Of Formation Potassium Hydroxide Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° −. Standard Heat Of Formation Potassium Hydroxide.
From www.slideserve.com
PPT Hess’s Law PowerPoint Presentation, free download ID6793985 Standard Heat Of Formation Potassium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t. Standard Heat Of Formation Potassium Hydroxide.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID314871 Standard Heat Of Formation Potassium Hydroxide 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t. Standard Heat Of Formation Potassium Hydroxide.
From www.chegg.com
Solved a) Calculate the standard heat of the neutralization Standard Heat Of Formation Potassium Hydroxide Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Heat Of Formation Potassium Hydroxide.
From www.slideshare.net
Heat of formation by reactions Standard Heat Of Formation Potassium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies.. Standard Heat Of Formation Potassium Hydroxide.
From mungfali.com
Enthalpies Of Formation Chart Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly. Standard Heat Of Formation Potassium Hydroxide.
From slideplayer.com
Chapter 10 “Thermochemistry” ppt download Standard Heat Of Formation Potassium Hydroxide 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t. Standard Heat Of Formation Potassium Hydroxide.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Potassium Hydroxide Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Gas phase heat. Standard Heat Of Formation Potassium Hydroxide.
From cewuaeqb.blob.core.windows.net
How Do You Work Out Standard Enthalpy Of Formation at Connie Stroud blog Standard Heat Of Formation Potassium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 +. Standard Heat Of Formation Potassium Hydroxide.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Heat Of Formation Potassium Hydroxide Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows. Standard Heat Of Formation Potassium Hydroxide.
From www.chegg.com
Solved Use and interpret standard heats of formation. (a) Standard Heat Of Formation Potassium Hydroxide Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: 193 rows in chemistry. Standard Heat Of Formation Potassium Hydroxide.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Formation Potassium Hydroxide Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,.. Standard Heat Of Formation Potassium Hydroxide.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Heat Of Formation Potassium Hydroxide Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: Gas phase heat capacity. Standard Heat Of Formation Potassium Hydroxide.
From www.numerade.com
Heat H2SO4 H2O Names Type of reaction Names potassium hydroxide Type Standard Heat Of Formation Potassium Hydroxide Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Gas phase heat capacity (shomate. Standard Heat Of Formation Potassium Hydroxide.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Potassium Hydroxide Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond energies. Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. Potassium hydroxide (koh) h 1 k 1 o 1 (cr) enthalpy reference temperature = t r = 298.15 k: 193 rows in chemistry and thermodynamics, the standard enthalpy. Standard Heat Of Formation Potassium Hydroxide.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly. Standard Heat Of Formation Potassium Hydroxide.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h Temperature and enthalpy of phase transitions and fusion, heat capacities, spectroscopic data, structures, bond. Standard Heat Of Formation Potassium Hydroxide.
From printablevascelomgm.z13.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Potassium Hydroxide Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t 2 h° − h° 298.15 = a*t + b*t 2 /2 + c*t 3 /3 + d*t 4 /4 − e/t + f − h 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Heat Of Formation Potassium Hydroxide.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Standard Heat Of Formation Potassium Hydroxide Potassium hydroxide is an inorganic compound with the formula k oh, and is commonly called caustic potash. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Gas phase heat capacity (shomate equation) c p ° = a + b*t + c*t 2 + d*t 3 + e/t. Standard Heat Of Formation Potassium Hydroxide.