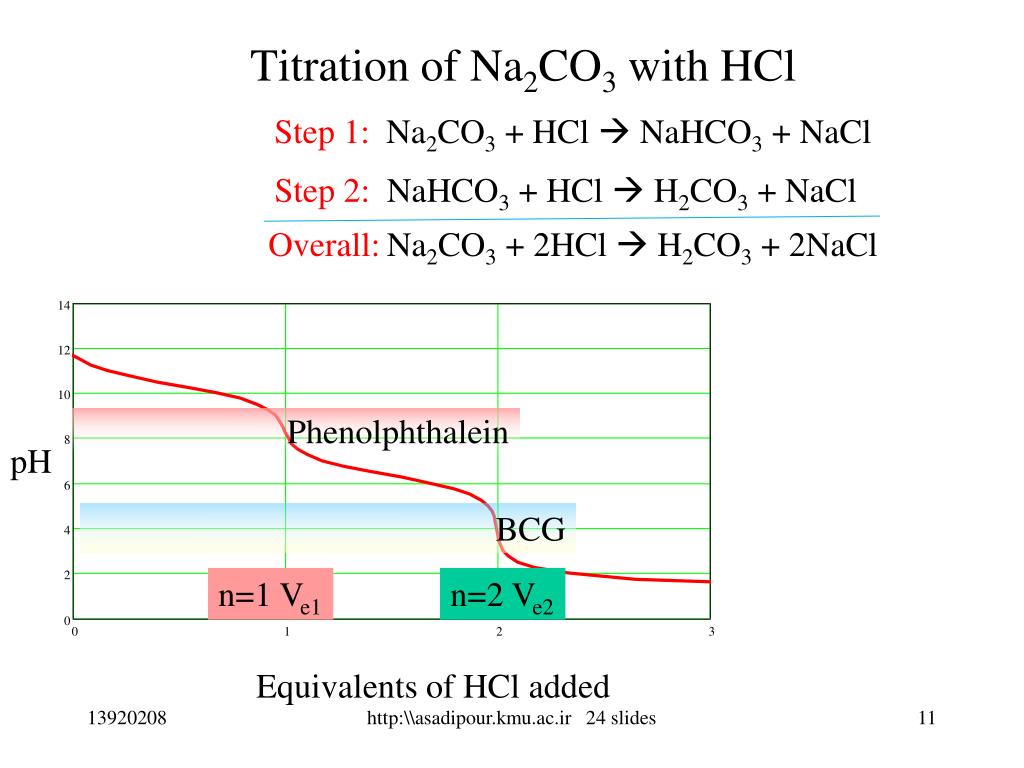
Titration Of H2So4 And Na2Co3 . Because the reaction between sodium carbonate and hydrochloric acid proceeds in. Hcl gradually reduces the alkalinity of the solution until the ph is 7. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Our titration calculator will help you never have to ask how do i calculate titrations? again. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color.
from www.slideserve.com
Hcl gradually reduces the alkalinity of the solution until the ph is 7. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Our titration calculator will help you never have to ask how do i calculate titrations? again. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the.
PPT Typical Applications of Neutralization Titrations Elemental
Titration Of H2So4 And Na2Co3 In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Our titration calculator will help you never have to ask how do i calculate titrations? again. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Hcl gradually reduces the alkalinity of the solution until the ph is 7.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Titration Of H2So4 And Na2Co3 European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. In each case, you start with 25. Titration Of H2So4 And Na2Co3.
From brainly.in
Determine the molarity of supplied Na2CO3 solution by titration by Titration Of H2So4 And Na2Co3 Hcl gradually reduces the alkalinity of the solution until the ph is 7. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. The example below demonstrates the technique to solve a titration problem for. Titration Of H2So4 And Na2Co3.
From stevenemahurino.blob.core.windows.net
Acid Base Titration Na2Co3 And Hcl at stevenemahurino blog Titration Of H2So4 And Na2Co3 Because the reaction between sodium carbonate and hydrochloric acid proceeds in. Our titration calculator will help you never have to ask how do i calculate titrations? again. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In each case, you start with 25 cm 3 of one of the. Titration Of H2So4 And Na2Co3.
From www.chegg.com
Solved After the titration of Na2CO3 (0.1M) with HCl using Titration Of H2So4 And Na2Co3 You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. Our titration calculator will help you never have to ask how do. Titration Of H2So4 And Na2Co3.
From www.studypool.com
SOLUTION The aim is to find the accurate concentration of sulphuric Titration Of H2So4 And Na2Co3 In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Hcl gradually reduces the alkalinity of the solution until the ph is 7. Because the reaction between. Titration Of H2So4 And Na2Co3.
From www.chegg.com
Solved 1. In the process of Na2CO3 titration against HCl, Titration Of H2So4 And Na2Co3 Hcl gradually reduces the alkalinity of the solution until the ph is 7. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2. Titration Of H2So4 And Na2Co3.
From www.studypool.com
SOLUTION Acid base titration objective to determine the concentration Titration Of H2So4 And Na2Co3 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. European pharmacopoeia describes the method for titer determination of h. Titration Of H2So4 And Na2Co3.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Of H2So4 And Na2Co3 European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Hcl gradually reduces the alkalinity of the solution until the ph is 7. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. Our titration calculator will. Titration Of H2So4 And Na2Co3.
From www.chegg.com
Solved Table 2 Back titration of H2SO4 with NaOH Mass of Titration Of H2So4 And Na2Co3 In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. Our. Titration Of H2So4 And Na2Co3.
From www.youtube.com
Type of Reaction for Na2CO3 + H2SO4 = Na2SO4 + CO2 + H2O YouTube Titration Of H2So4 And Na2Co3 European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Our titration calculator will help you never have to ask how do i calculate titrations? again. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. Hcl gradually reduces the alkalinity of the solution until. Titration Of H2So4 And Na2Co3.
From www.slideserve.com
PPT Typical Applications of Neutralization Titrations Elemental Titration Of H2So4 And Na2Co3 Because the reaction between sodium carbonate and hydrochloric acid proceeds in. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. Our titration calculator will help you never have to ask. Titration Of H2So4 And Na2Co3.
From www.youtube.com
Determination of Na2CO3 and NaOH in a mixture by titration YouTube Titration Of H2So4 And Na2Co3 In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration. Titration Of H2So4 And Na2Co3.
From www.studypool.com
SOLUTION The aim is to find the accurate concentration of sulphuric Titration Of H2So4 And Na2Co3 Hcl gradually reduces the alkalinity of the solution until the ph is 7. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Because the reaction between. Titration Of H2So4 And Na2Co3.
From www.youtube.com
titration of sodium carbonate with HCI titration of Na2CO3 Vs HCI Titration Of H2So4 And Na2Co3 Because the reaction between sodium carbonate and hydrochloric acid proceeds in. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. The example below demonstrates the technique. Titration Of H2So4 And Na2Co3.
From www.youtube.com
''Na2CO3 & NaCl'' Mixture Analysis Titration Application Titration Of H2So4 And Na2Co3 Because the reaction between sodium carbonate and hydrochloric acid proceeds in. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide.. Titration Of H2So4 And Na2Co3.
From dxohazwro.blob.core.windows.net
Titration Hcl And Na2Co3 at Madeline Arnold blog Titration Of H2So4 And Na2Co3 Hcl gradually reduces the alkalinity of the solution until the ph is 7. Our titration calculator will help you never have to ask how do i calculate titrations? again. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric. Titration Of H2So4 And Na2Co3.
From www.youtube.com
How to Balance H2SO4 + Na2CO3 = Na2SO4 + CO2 + H2O YouTube Titration Of H2So4 And Na2Co3 You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Hcl gradually reduces the alkalinity of the. Titration Of H2So4 And Na2Co3.
From www.numerade.com
SOLVED Na2CO3 reacts with H2SO4 to give respective salt water and Titration Of H2So4 And Na2Co3 European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. Hcl gradually reduces the alkalinity of the solution until the ph is 7. In each case, you start with 25 cm 3 of one of. Titration Of H2So4 And Na2Co3.
From www.chegg.com
Solved Titration Lab Report Titration of Na2CO3 with HCl Titration Of H2So4 And Na2Co3 Because the reaction between sodium carbonate and hydrochloric acid proceeds in. Hcl gradually reduces the alkalinity of the solution until the ph is 7. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. The example below demonstrates the technique to. Titration Of H2So4 And Na2Co3.
From www.numerade.com
SOLVED Given the balanced equation H2SO4 + Na2CO3 > Na2SO4 + H2O Titration Of H2So4 And Na2Co3 Because the reaction between sodium carbonate and hydrochloric acid proceeds in. Hcl gradually reduces the alkalinity of the solution until the ph is 7. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. You can use the technique of titration to determine the concentration of a sodium carbonate solution using. Titration Of H2So4 And Na2Co3.
From byjus.com
in the titration of Na2CO3 by HCl using methyl orange indicator Titration Of H2So4 And Na2Co3 In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. Our titration calculator will help you never have to ask how do i calculate titrations? again. European pharmacopoeia describes the method for titer determination of h 2 so 4 using. Titration Of H2So4 And Na2Co3.
From www.scribd.com
1 Estimation of Na2CO3 and NaOH in A Mixture Using HCL PDF Titration Of H2So4 And Na2Co3 European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Hcl gradually reduces the alkalinity of the solution until the ph is 7. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. Because the reaction between. Titration Of H2So4 And Na2Co3.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Titration Of H2So4 And Na2Co3 Hcl gradually reduces the alkalinity of the solution until the ph is 7. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or. Titration Of H2So4 And Na2Co3.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Titration Of H2So4 And Na2Co3 You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In each case, you start with 25 cm 3 of one. Titration Of H2So4 And Na2Co3.
From www.studypool.com
SOLUTION Titration na2co3 vs hcl Studypool Titration Of H2So4 And Na2Co3 European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. Our titration calculator will help you never. Titration Of H2So4 And Na2Co3.
From www.youtube.com
ACIDBASE TITRATION between H2SO4 and Na2CO3 Chemistry Practical Titration Of H2So4 And Na2Co3 You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Because the reaction between sodium carbonate and hydrochloric acid proceeds in.. Titration Of H2So4 And Na2Co3.
From stevenemahurino.blob.core.windows.net
Acid Base Titration Na2Co3 And Hcl at stevenemahurino blog Titration Of H2So4 And Na2Co3 Because the reaction between sodium carbonate and hydrochloric acid proceeds in. Our titration calculator will help you never have to ask how do i calculate titrations? again. Hcl gradually reduces the alkalinity of the solution until the ph is 7. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric. Titration Of H2So4 And Na2Co3.
From www.numerade.com
SOLVED Aqueous solutions of sodium bicarbonate and sulfuric acid react Titration Of H2So4 And Na2Co3 You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In each case, you start with 25 cm 3 of one. Titration Of H2So4 And Na2Co3.
From www.studypool.com
SOLUTION Acid base titration objective to determine the concentration Titration Of H2So4 And Na2Co3 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Hcl gradually reduces the alkalinity of the solution until the ph is 7. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. You can use the technique of titration to determine the concentration of a sodium carbonate solution. Titration Of H2So4 And Na2Co3.
From eodev.com
Na2CO3, H2SO4 , Mg(PO4) C nin. S nin P ninAltı çizili (altlarında Titration Of H2So4 And Na2Co3 Because the reaction between sodium carbonate and hydrochloric acid proceeds in. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Hcl gradually reduces the alkalinity of the solution until the ph is 7. You can use the technique of titration to determine the concentration of a sodium carbonate solution. Titration Of H2So4 And Na2Co3.
From www.youtube.com
Na2CO3 and H2SO4 Acid Base Titration YouTube Titration Of H2So4 And Na2Co3 In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. Our titration calculator will help you never have to ask how do i calculate titrations? again. You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric. Titration Of H2So4 And Na2Co3.
From www.youtube.com
Double Indicator Titration (Part 1) NaOH and Na2CO3 mixture YouTube Titration Of H2So4 And Na2Co3 European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Our titration calculator will help you never have to ask how do i calculate titrations? again. Hcl gradually reduces the alkalinity of the solution until the ph is 7. In each case, you start with 25. Titration Of H2So4 And Na2Co3.
From www.numerade.com
SOLVED Calculate the Normality of the H2SO4 solution if 41.59 mL is Titration Of H2So4 And Na2Co3 Hcl gradually reduces the alkalinity of the solution until the ph is 7. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. The example below demonstrates. Titration Of H2So4 And Na2Co3.
From www.studypool.com
SOLUTION Acid base titration objective to determine the concentration Titration Of H2So4 And Na2Co3 You can use the technique of titration to determine the concentration of a sodium carbonate solution using a solution with a known concentration of hydrochloric acid, or vice versa. Because the reaction between sodium carbonate and hydrochloric acid proceeds in. In each case, you start with 25 cm 3 of one of the solutions in the flask, and the. Our. Titration Of H2So4 And Na2Co3.
From www.gutefrage.net
Titration Na2CO3 + H2SO4? (molare Masse) Titration Of H2So4 And Na2Co3 The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. European pharmacopoeia describes the method for titer determination of h 2 so 4 using na 2 co 3 with colorimetric titration using a color. Hcl gradually reduces the alkalinity of the solution until the ph is 7. Because the reaction. Titration Of H2So4 And Na2Co3.