Magnesium Chloride Number Of Formula Units . The formula of magnesium chloride is mgcl2. Your solution’s ready to go! There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. Number of formula units = (mass of substance). 1.58 * 10^ (23) you know that. Let us start by calculating the formula mass of sodium chloride (nacl). The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. Part c how many formula units make up 19.8 g. Calculate the molar mass of magnesium chloride (mgcl2). The answer is rounded to three sig figs.
from www.numerade.com
Your solution’s ready to go! The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. Part c how many formula units make up 19.8 g. The answer is rounded to three sig figs. Let us start by calculating the formula mass of sodium chloride (nacl). Calculate the molar mass of magnesium chloride (mgcl2). 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. The formula of magnesium chloride is mgcl2. There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride.
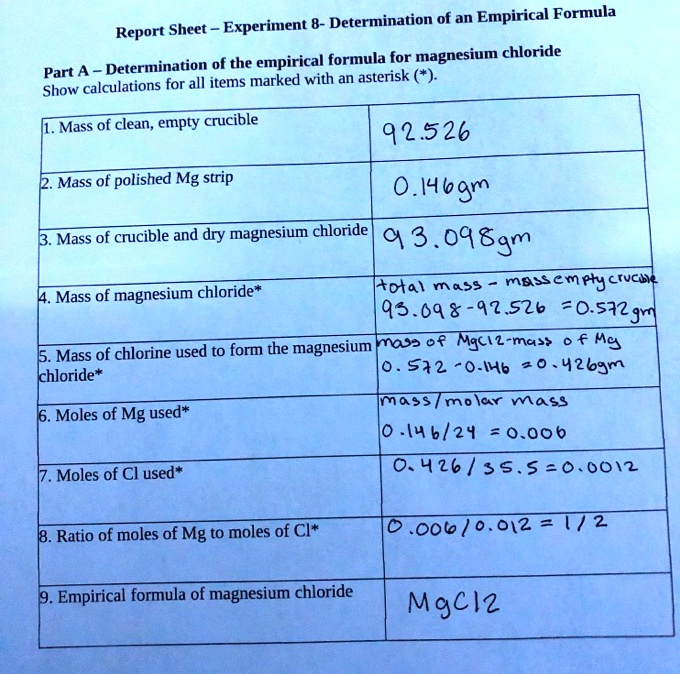
SOLVED Experiment Determination of an Empirical Formula Report Sheet Determination of the
Magnesium Chloride Number Of Formula Units 1.58 * 10^ (23) you know that. Calculate the molar mass of magnesium chloride (mgcl2). The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). The formula of magnesium chloride is mgcl2. There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. Let us start by calculating the formula mass of sodium chloride (nacl). 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. Your solution’s ready to go! Number of formula units = (mass of substance). 1.58 * 10^ (23) you know that. The answer is rounded to three sig figs. Part c how many formula units make up 19.8 g.
From www.science-revision.co.uk
Introduction to ionic bonding Magnesium Chloride Number Of Formula Units Number of formula units = (mass of substance). The answer is rounded to three sig figs. If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). The formula of magnesium chloride is mgcl2. This formula mass is the sum of the atomic masses of. Magnesium Chloride Number Of Formula Units.
From astonishingceiyrs.blogspot.com
Magnesium Chloride Formula astonishingceiyrs Magnesium Chloride Number Of Formula Units Your solution’s ready to go! The answer is rounded to three sig figs. Calculate the molar mass of magnesium chloride (mgcl2). The formula of magnesium chloride is mgcl2. There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. To. Magnesium Chloride Number Of Formula Units.
From www.chegg.com
Solved Part C How many formula units make up 26.2 g of Magnesium Chloride Number Of Formula Units If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). Let us start by calculating the formula mass of sodium chloride (nacl). This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. Part c how many. Magnesium Chloride Number Of Formula Units.
From www.chegg.com
Solved Part C How many formula units make up 13.6 g of Magnesium Chloride Number Of Formula Units Your solution’s ready to go! Number of formula units = (mass of substance). The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. Part c how many formula units make up 19.8 g. The answer is rounded to three sig figs. 1.58 * 10^ (23) you know that. If. Magnesium Chloride Number Of Formula Units.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Magnesium Chloride Number Of Formula Units There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: Let us start by calculating the formula mass of sodium chloride (nacl). Part c how many formula units make up 19.8 g. The answer is rounded to three sig figs. This. Magnesium Chloride Number Of Formula Units.
From www.youtube.com
Number of Ions in MgCl2 Magnesium chloride YouTube Magnesium Chloride Number Of Formula Units Part c how many formula units make up 19.8 g. The answer is rounded to three sig figs. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: Calculate the molar mass of magnesium chloride (mgcl2). If i understand your question correctly, you can simply divide the total number of formula units of. Magnesium Chloride Number Of Formula Units.
From www.knowledgeboat.com
Chapter 2 Chemical Bonding Selina Solutions Concise Chemistry Class 10 ICSE KnowledgeBoat Magnesium Chloride Number Of Formula Units To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: Number of formula units = (mass of substance). If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). Part c how many formula units make up 19.8. Magnesium Chloride Number Of Formula Units.
From www.youtube.com
Empirical Formula of Magnesium Chloride LAB YouTube Magnesium Chloride Number Of Formula Units Calculate the molar mass of magnesium chloride (mgcl2). The answer is rounded to three sig figs. Number of formula units = (mass of substance). Your solution’s ready to go! 1.58 * 10^ (23) you know that. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: The answer is rounded to three sig. Magnesium Chloride Number Of Formula Units.
From www.numerade.com
SOLVED How many formula units make up 20.0 gg of magnesium chloride (MgCl2MgCl2)? Magnesium Chloride Number Of Formula Units Your solution’s ready to go! Number of formula units = (mass of substance). 1.58 * 10^ (23) you know that. If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). The answer is rounded to three sig figs, the number of sig figs you. Magnesium Chloride Number Of Formula Units.
From askfilo.com
rite. the chemical formula of ) magnesium chloride ⇒MgCl2 ⇒ IS Ealciu.. Magnesium Chloride Number Of Formula Units The answer is rounded to three sig figs. 1.58 * 10^ (23) you know that. The formula of magnesium chloride is mgcl2. Calculate the molar mass of magnesium chloride (mgcl2). To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: Let us start by calculating the formula mass of sodium chloride (nacl). There. Magnesium Chloride Number Of Formula Units.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Magnesium Chloride Number Of Formula Units 1.58 * 10^ (23) you know that. The answer is rounded to three sig figs. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. Number of formula units = (mass of substance). This. Magnesium Chloride Number Of Formula Units.
From www.numerade.com
SOLVED Calculate the number of moles of Cl atoms in 3.01×10^24 formula units of magnesium Magnesium Chloride Number Of Formula Units There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. The formula of magnesium chloride is mgcl2. If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). 1.58 * 10^ (23) you know that. The answer is rounded to three sig figs.. Magnesium Chloride Number Of Formula Units.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Magnesium Chloride Number Of Formula Units 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: Number of formula units = (mass of substance). There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. 1.58 * 10^ (23). Magnesium Chloride Number Of Formula Units.
From oneclass.com
OneClass How many formula units make up 29.2 g of magnesium chloride (MgCl2)? Magnesium Chloride Number Of Formula Units This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: There are 2.52 ×1023. Magnesium Chloride Number Of Formula Units.
From tutorsuhu.com
What Is The Molecular Structure Of Magnesium Chloride Tutor Suhu Magnesium Chloride Number Of Formula Units 1.58 * 10^ (23) you know that. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: Calculate the molar mass of magnesium chloride (mgcl2). The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. Let us start by calculating the formula. Magnesium Chloride Number Of Formula Units.
From www.numerade.com
SOLVED How many formula units make up 36.4 g of magnesium chloride (MgCl2)? Express the number Magnesium Chloride Number Of Formula Units The formula of magnesium chloride is mgcl2. If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. Number of formula units = (mass of substance).. Magnesium Chloride Number Of Formula Units.
From ar.inspiredpencil.com
Magnesium Chloride Structure Magnesium Chloride Number Of Formula Units The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. Calculate the molar mass of magnesium chloride (mgcl2). This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. Let us start by calculating the formula mass of sodium chloride (nacl). The. Magnesium Chloride Number Of Formula Units.
From molekula.com
Purchase Magnesium chloride anhydrous [7786303] online • Catalog • Molekula Group Magnesium Chloride Number Of Formula Units The answer is rounded to three sig figs. If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). Calculate the molar mass of magnesium chloride (mgcl2). The answer is rounded to three sig figs, the number of sig figs you have for the mass. Magnesium Chloride Number Of Formula Units.
From www.youtube.com
Write the chemical formula of the following compounds (a)Magnesium Chloride (b) Calcium oxide Magnesium Chloride Number Of Formula Units This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. 1.58 * 10^ (23) you know that. There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. The formula of magnesium chloride is mgcl2. Your solution’s ready to go! The answer is rounded to three sig figs, the number of. Magnesium Chloride Number Of Formula Units.
From www.chegg.com
Chemistry Archive March 03, 2017 Magnesium Chloride Number Of Formula Units The formula of magnesium chloride is mgcl2. There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. Calculate the molar mass of magnesium chloride (mgcl2). Your solution’s ready to go! To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: 1.58 * 10^ (23) you know that. If i understand your. Magnesium Chloride Number Of Formula Units.
From www.youtube.com
(a) Show the formation of magnesium chloride and sodium chloride by transfer YouTube Magnesium Chloride Number Of Formula Units This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: The formula of magnesium chloride is mgcl2. If i understand your question correctly, you can simply divide the total number of formula units of the. Magnesium Chloride Number Of Formula Units.
From www.pngwing.com
Lewis structure Magnesium chloride Electron Diagram, Dot, text, trademark, rectangle png PNGWing Magnesium Chloride Number Of Formula Units Number of formula units = (mass of substance). This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. The answer is rounded to three sig figs. 1.58 * 10^ (23) you know that. Let us start by calculating the formula mass of sodium chloride (nacl). There are 2.52 ×1023 formula units in. Magnesium Chloride Number Of Formula Units.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Chloride Number Of Formula Units Part c how many formula units make up 19.8 g. Let us start by calculating the formula mass of sodium chloride (nacl). The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula:. Magnesium Chloride Number Of Formula Units.
From www.chegg.com
Solved How many formula units make up 11.8 g of magnesium Magnesium Chloride Number Of Formula Units Number of formula units = (mass of substance). To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). This formula mass is the sum of the atomic. Magnesium Chloride Number Of Formula Units.
From www.chegg.com
Solved Part C How many formula units make up 16.8 g of Magnesium Chloride Number Of Formula Units To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. 1.58 * 10^ (23) you know that. The formula of magnesium chloride is mgcl2. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine. Magnesium Chloride Number Of Formula Units.
From jbdizkkrki.blogspot.com
Magnesium Chloride Formula Unit The Structure Of Matter Section 1 Compounds And Formula Magnesium Chloride Number Of Formula Units 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: The formula of magnesium chloride is mgcl2. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine. Magnesium Chloride Number Of Formula Units.
From www.numerade.com
SOLVED Experiment Determination of an Empirical Formula Report Sheet Determination of the Magnesium Chloride Number Of Formula Units Part c how many formula units make up 19.8 g. 1.58 * 10^ (23) you know that. The answer is rounded to three sig figs. Calculate the molar mass of magnesium chloride (mgcl2). Your solution’s ready to go! There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. The formula of magnesium chloride is mgcl2. This formula mass. Magnesium Chloride Number Of Formula Units.
From solvedlib.com
How many formula units make up 21.8 g of magnesium ch… SolvedLib Magnesium Chloride Number Of Formula Units The answer is rounded to three sig figs. Part c how many formula units make up 19.8 g. Let us start by calculating the formula mass of sodium chloride (nacl). 1.58 * 10^ (23) you know that. Your solution’s ready to go! Number of formula units = (mass of substance). This formula mass is the sum of the atomic masses. Magnesium Chloride Number Of Formula Units.
From inci.guide
Magnesium Chloride Ingredient INCIGuide Magnesium Chloride Number Of Formula Units Let us start by calculating the formula mass of sodium chloride (nacl). To find the number of formula units in 19.0 g of magnesium chloride (mgcl2), use the formula: The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. Calculate the molar mass of magnesium chloride (mgcl2). Part c. Magnesium Chloride Number Of Formula Units.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Chloride Number Of Formula Units The answer is rounded to three sig figs. Let us start by calculating the formula mass of sodium chloride (nacl). If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). 1.58 * 10^ (23) you know that. Part c how many formula units make. Magnesium Chloride Number Of Formula Units.
From oneclass.com
OneClass â ¼ Part C How many formula units make up 33.8 g of magnesium chloride (MgC2)? Express Magnesium Chloride Number Of Formula Units If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). Number of formula units = (mass of substance). Your solution’s ready to go! Let us start by calculating the formula mass of sodium chloride (nacl). 2.37 * 10^ (23) the first thing to do. Magnesium Chloride Number Of Formula Units.
From www.alamy.com
3D image of Magnesium chloride skeletal formula molecular chemical structure of Magnesium Chloride Number Of Formula Units 1.58 * 10^ (23) you know that. The answer is rounded to three sig figs. If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). Calculate the molar mass of magnesium chloride (mgcl2). The formula of magnesium chloride is mgcl2. There are 2.52 ×1023. Magnesium Chloride Number Of Formula Units.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Magnesium Chloride Number Of Formula Units 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. Your solution’s ready to go! The answer is rounded to three sig figs, the number of sig figs you have for the mass of the sample. Calculate the molar mass of magnesium chloride (mgcl2). There are 2.52 ×1023 formula units in. Magnesium Chloride Number Of Formula Units.
From cymitquimica.com
Magnesium chloride hexahydrate, BP, Ph. Eur. grade Magnesium Chloride Number Of Formula Units This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium chloride to. Part c how many formula units make up 19.8 g. If i understand your question correctly, you can simply divide the total number. Magnesium Chloride Number Of Formula Units.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Magnesium Chloride Number Of Formula Units There are 2.52 ×1023 formula units in 39.8 g of magnesium chloride. If i understand your question correctly, you can simply divide the total number of formula units of the compound by avogadro's number (6.02x10 23 formula units/mol). Your solution’s ready to go! 2.37 * 10^ (23) the first thing to do here is to convert the mass of magnesium. Magnesium Chloride Number Of Formula Units.