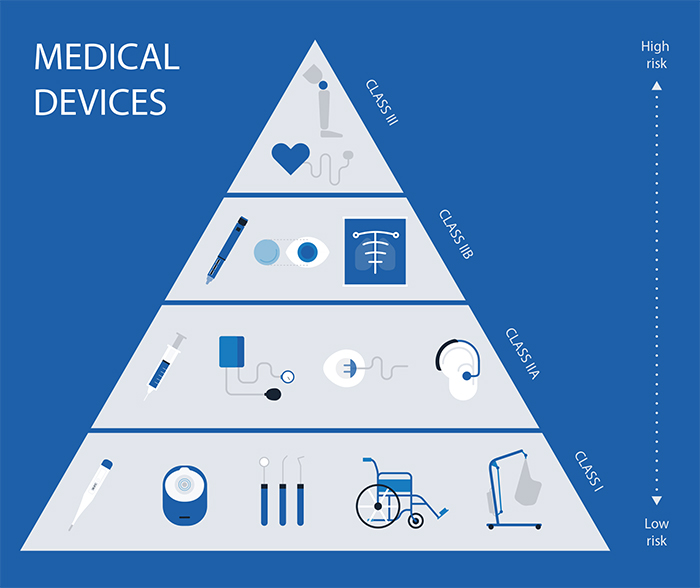
Medical Device Definition In Eu . If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. For the purposes of this regulation, the following definitions apply: Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. In the european union (eu) they must undergo a conformity. (1) ‘medical device’ means any instrument,. (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. Medical devices are products or equipment intended for a medical purpose. For the purposes of this regulation, the following definitions apply:
from laegemiddelstyrelsen.dk
If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. For the purposes of this regulation, the following definitions apply: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. (1) ‘medical device’ means any instrument,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. For the purposes of this regulation, the following definitions apply: In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose.
Medical devices
Medical Device Definition In Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument,. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For the purposes of this regulation, the following definitions apply: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Medical devices are products or equipment intended for a medical purpose. (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. In the european union (eu) they must undergo a conformity. For the purposes of this regulation, the following definitions apply:
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Medical Device Definition In Eu (1) ‘medical device’ means any instrument,. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. In the. Medical Device Definition In Eu.
From omcmedical.com
Exceptional Use of Medical Devices in UK & EU OMC Medical Medical Device Definition In Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a. Medical Device Definition In Eu.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I more involved Medical Device Definition In Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Medical devices are products or equipment intended for a medical purpose. For the purposes of this regulation, the following definitions apply: A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. (1) ‘medical. Medical Device Definition In Eu.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Medical Device Definition In Eu In the european union (eu) they must undergo a conformity. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. Medical devices are products or equipment intended for a medical purpose. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument,. (1) ‘medical. Medical Device Definition In Eu.
From medicaldevicehq.com
Medical device design control terminology Medical Device HQ 1 Medical Device Definition In Eu In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. If you are a manufacturer, authorised representative, importer or distributor of medical. Medical Device Definition In Eu.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Medical Device Definition In Eu Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,.. Medical Device Definition In Eu.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Medical Device Definition In Eu (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of. Medical Device Definition In Eu.
From www.presentationeze.com
EU Medical Device Classification per the EU Directives PresentationEZE Medical Device Definition In Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument,. For the purposes of this regulation, the following definitions apply: Medical devices are products or equipment. Medical Device Definition In Eu.
From www.qualitymeddev.com
Medical Device Definition according to EU MDR 2017/745 Medical Device Definition In Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For the purposes of this regulation, the following definitions apply: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Medical devices are products or equipment intended for a medical purpose. If. Medical Device Definition In Eu.
From www2.deloitte.com
The new European Union medical devices regulation Deloitte Life Sciences and Healthcare Medical Device Definition In Eu (1) ‘medical device’ means any instrument,. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or. Medical Device Definition In Eu.
From operonstrategist.com
Medical Device Classification in Europe Your Guide to Navigating Compliance Operon Strategist Medical Device Definition In Eu In the european union (eu) they must undergo a conformity. For the purposes of this regulation, the following definitions apply: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. If you. Medical Device Definition In Eu.
From emmainternational.com
Classifying Medical Devices under EU MDR Medical Device Definition In Eu (1) ‘medical device’ means any instrument,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. Regulation (eu) 2017/745. Medical Device Definition In Eu.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Medical Device Definition In Eu In the european union (eu) they must undergo a conformity. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for. Medical Device Definition In Eu.
From www.universalmedica.com
Key Aspects of New EU Medical Devices Regulation (EU 2017/745). Universal Medica Medical Device Definition In Eu If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. For the purposes of this regulation, the following definitions apply: In the european union (eu) they must undergo a conformity. Medical. Medical Device Definition In Eu.
From clin-r.com
EU MDR how to structure your Medical Device Technical Document Clin R Medical Device Definition In Eu In the european union (eu) they must undergo a conformity. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. (1) ‘medical device’ means any instrument,. Medical devices are products or equipment intended for a medical purpose. (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. For the. Medical Device Definition In Eu.
From www.jamasoftware.com
What the New Medical Device Regulations (EU MDR) Mean for You Jama Software Medical Device Definition In Eu Medical devices are products or equipment intended for a medical purpose. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. In the european union (eu) they must undergo a conformity. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical.. Medical Device Definition In Eu.
From pepgra.com
Medical Device Classification In The European Union pepgra Medical Device Definition In Eu Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For the purposes of this regulation, the following definitions apply: If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. (1) ‘medical device’ means ‘medical device’ as defined in. Medical Device Definition In Eu.
From www.ondrugdelivery.com
THE NEW EU MEDICAL DEVICE REGULATIONS IMPLICATIONS FOR INHALATION DEVICES ONdrugDelivery Medical Device Definition In Eu If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. For the purposes of this regulation, the following definitions apply: Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a. Medical Device Definition In Eu.
From www.pinterest.com
What is a Medical Device? (Official definition for EU, USA, China, Brazil) Medical, Medical Medical Device Definition In Eu For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. Medical Device Definition In Eu.
From credevo.com
Europe Medical Device Market Approval Credevo Articles Medical Device Definition In Eu In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. For the purposes of this regulation, the following definitions apply: For the purposes of this regulation, the following definitions apply: A. Medical Device Definition In Eu.
From medidee.com
[ARTICLE] Combination Products Similarities and Differences of EU and US Regulations Medidee Medical Device Definition In Eu If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. For the purposes of this regulation, the following definitions apply: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. For the purposes of this regulation, the following definitions apply: A. Medical Device Definition In Eu.
From omcmedical.com
EU Classification of Medical Devices OMC Medical Medical Device Definition In Eu For the purposes of this regulation, the following definitions apply: The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For. Medical Device Definition In Eu.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Definition In Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Medical devices are products or equipment intended for a medical purpose. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. Regulation (eu) 2017/745 of the european parliament and of the. Medical Device Definition In Eu.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU MDR Requirements Oriel Medical Device Definition In Eu The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. (1) ‘medical device’ means any instrument,. For the purposes of this. Medical Device Definition In Eu.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Definition In Eu For the purposes of this regulation, the following definitions apply: A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu) they must undergo a conformity. (1). Medical Device Definition In Eu.
From laegemiddelstyrelsen.dk
Medical devices Medical Device Definition In Eu Medical devices are products or equipment intended for a medical purpose. (1) ‘medical device’ means any instrument,. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. In the european union (eu) they. Medical Device Definition In Eu.
From easymedicaldevice.com
EU MDR 2017/745 Transition timeline [Medical Device Regulation] Medical Device Definition In Eu For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. (1) ‘medical device’ means any instrument,. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. For the purposes of this regulation, the following definitions apply: Medical. Medical Device Definition In Eu.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Definition In Eu (1) ‘medical device’ means any instrument,. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. Medical. Medical Device Definition In Eu.
From medicaldevicehq.com
Different classifications rules for medical device software An introduction Medical Device Definition In Eu For the purposes of this regulation, the following definitions apply: If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. (1) ‘medical device’ means any instrument,. (1) ‘medical device’ means ‘medical. Medical Device Definition In Eu.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Device Definition In Eu (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. Medical devices are products or equipment intended for a medical purpose. For the purposes of this regulation, the following definitions apply: If you are a manufacturer, authorised representative, importer. Medical Device Definition In Eu.
From www.i3cglobal.com
EU Medical Device Classification Examples and Rules Medical Device Definition In Eu Medical devices are products or equipment intended for a medical purpose. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. The medical device regulation (mdr), which. Medical Device Definition In Eu.
From www.arenasolutions.com
Medical Device Definition Arena Medical Device Definition In Eu For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. For the purposes of this regulation, the following. Medical Device Definition In Eu.
From www.freyrsolutions.com
Medical Devices and CE Marking Process under the EU MDR Freyr Global Regulatory Solutions Medical Device Definition In Eu If you are a manufacturer, authorised representative, importer or distributor of medical devices in the eu, or a regulatory affairs or. In the european union (eu) they must undergo a conformity. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument,. A medical device is any product used for medical purposes, including diagnosis, prevention,. Medical Device Definition In Eu.
From www.motaword.com
EU Medical Device Regulation What Do You Need To Know? Medical Device Definition In Eu A medical device is any product used for medical purposes, including diagnosis, prevention, treatment, investigation or changes to anatomy,. In the european union (eu) they must undergo a conformity. (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending. Medical Device Definition In Eu.
From www.greenlight.guru
Medical Device Adverse Event Reporting Regulations EU vs. US Medical Device Definition In Eu (1) ‘medical device’ means ‘medical device’ as defined in point (1) of. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any instrument,. The medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for medical. Medical devices are products or equipment intended for a medical purpose. In the european. Medical Device Definition In Eu.