Paracetamol Naprex Iv Drug Study . This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h.
from www.studocu.com
No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than.
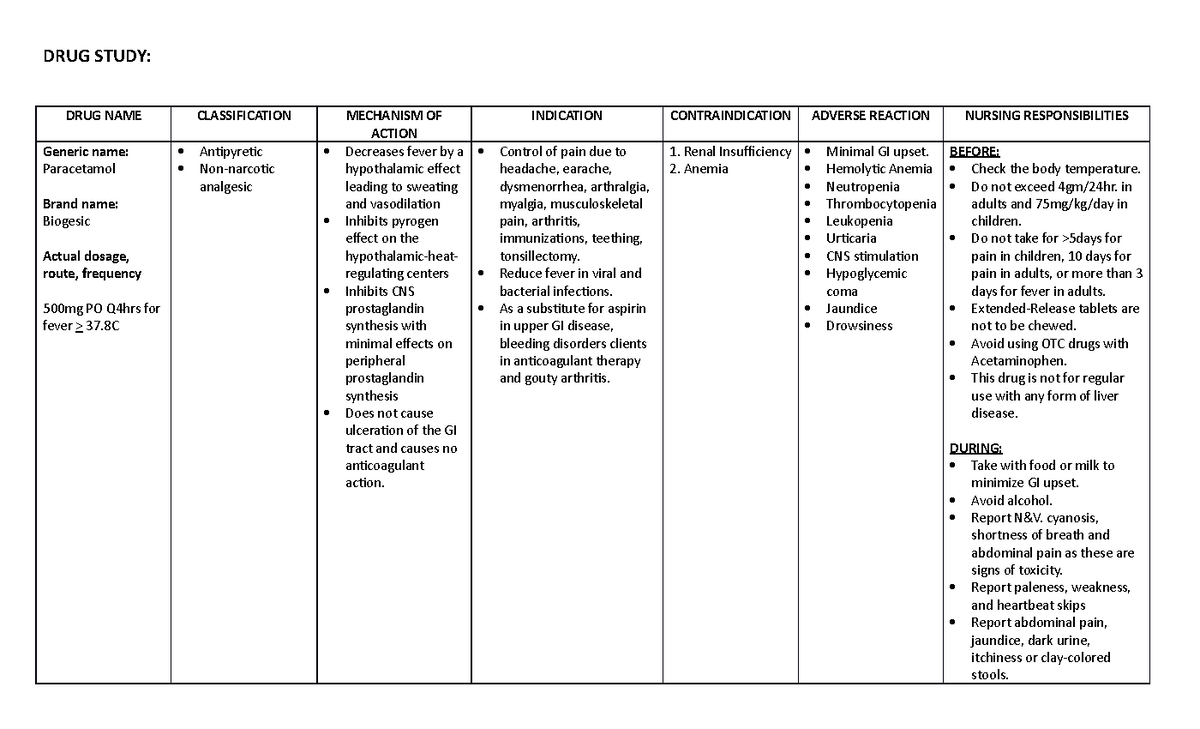
DRUG Study Paracetamol DRUG STUDY DRUG NAME CLASSIFICATION
Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the.
From www.studocu.com
Paracetamol Drug Study Drug Order Mechanism of Action Indication Paracetamol Naprex Iv Drug Study No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
DRUG STUDY Paracetamol PDF Clinical Medicine Pharmacology Paracetamol Naprex Iv Drug Study Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Drug Study Paracetamol PDF Analgesic Dose (Biochemistry) Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for. Paracetamol Naprex Iv Drug Study.
From www.studocu.com
Drug study paracetamol GENERIC NAME Paracetamol MECHANISM OF ACTION Paracetamol Naprex Iv Drug Study Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.studocu.com
Drug Profile Paracetamol Drug name Approved or generic? Paracetamol Paracetamol Naprex Iv Drug Study Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.unilab.com.ph
Naprex Solution Analgesic & Antipyretic Medicine Unilab Paracetamol Naprex Iv Drug Study Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.studocu.com
DRUG Study Paracetamol DRUG STUDY Name of Patient Baby Pearl Date of Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Drug Study Paracetamol PDF Analgesic Medical Treatments Paracetamol Naprex Iv Drug Study Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. No randomized study has been conducted. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Paracetamol Drug Study PDF Dose (Biochemistry) Chemistry Paracetamol Naprex Iv Drug Study No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Drug Study Paracetamol Paracetamol Naprex Iv Drug Study Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.studypool.com
SOLUTION DRUG STUDIES (Paracetamol) docx Studypool Paracetamol Naprex Iv Drug Study Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.studocu.com
DRUG Study Paracetamol 1g (Infulgan) IV Q8H x 3 more doses Generic Paracetamol Naprex Iv Drug Study No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.studocu.com
Drug Study Paracetamol DRUG STUDY GENERIC NAME Paracetamol MECHANISM Paracetamol Naprex Iv Drug Study Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Paracetamol Drug Study Paracetamol Naprex Iv Drug Study Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.studocu.com
A drug study of paracetamol Pharmacy Studocu Paracetamol Naprex Iv Drug Study Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Drug Study Acetaminophen, Paracetamol Paracetamol Naprex Iv Drug Study No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Drug Study Paracetamol PDF Medicinal Chemistry Health Sciences Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.studocu.com
DRUG Study Paracetamol DRUG STUDY DRUG NAME CLASSIFICATION Paracetamol Naprex Iv Drug Study Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.studocu.com
VAP Paracetamol Series of Pharmacology drug study in the third year Paracetamol Naprex Iv Drug Study No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Paracetamol PDF Drugs Health Sciences Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Naprex Drug Study PDF Clinical Medicine Medical Specialties Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
DRUG STUDY Biogesic Paracetamol Paracetamol Naprex Iv Drug Study No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.studypool.com
SOLUTION Paracetamol drug study Studypool Paracetamol Naprex Iv Drug Study No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.studocu.com
Drug study on paracetamol (classification, action, indications Paracetamol Naprex Iv Drug Study Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Paracetamol Drugstudy PDF Paracetamol Naprex Iv Drug Study Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
DRUG+STUDY Naprex PDF Fever Analgesic Paracetamol Naprex Iv Drug Study Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Paracetamol Drug Study Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Drug Study Paracetamol Analgesic Pharmacology Paracetamol Naprex Iv Drug Study Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Drug Study Paracetamol PDF Fever Analgesic Paracetamol Naprex Iv Drug Study Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Drug Study Paracetamol PDF Fever Medical Specialties Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted. Paracetamol Naprex Iv Drug Study.
From www.studypool.com
SOLUTION DRUG STUDIES (Paracetamol) docx Studypool Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Paracetamol Drug Study PDF Dose (Biochemistry) Chemistry Paracetamol Naprex Iv Drug Study Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. Useful in conditions where a rapid predictable blood level is. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. This safety study indicates that iv paracetamol can. Paracetamol Naprex Iv Drug Study.
From pt.scribd.com
Paracetamol Drug Study Drugs Pharmacology Paracetamol Naprex Iv Drug Study Useful in conditions where a rapid predictable blood level is. This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.
From www.studypool.com
SOLUTION Paracetamol drug study Studypool Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number of patients achieving at least 50% pain relief over 4 h. No randomized study has been conducted. Paracetamol Naprex Iv Drug Study.
From www.scribd.com
Paracetamol Drug Study PDF Paracetamol Naprex Iv Drug Study This safety study indicates that iv paracetamol can be administered in paediatric patients with a shorter infusion time than. No randomized study has been conducted to investigate the use of intravenous paracetamol (acetaminophen, apap) for the. Useful in conditions where a rapid predictable blood level is. Nine studies provided data on the first time period of the primary outcome, number. Paracetamol Naprex Iv Drug Study.