Reaction Between Chlorine And Alcohol . The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Chemical reactions in alcohols occur mainly at the functional. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Alcohol + sodium → sodium alkoxide + hydrogen. Describe the result of the oxidation of a secondary alcohol. Describe the result of the oxidation of a primary alcohol. Thionyl chloride creates an intermediate. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Water is expelled to generate a carbocation, and the cation.
from wisc.pb.unizin.org
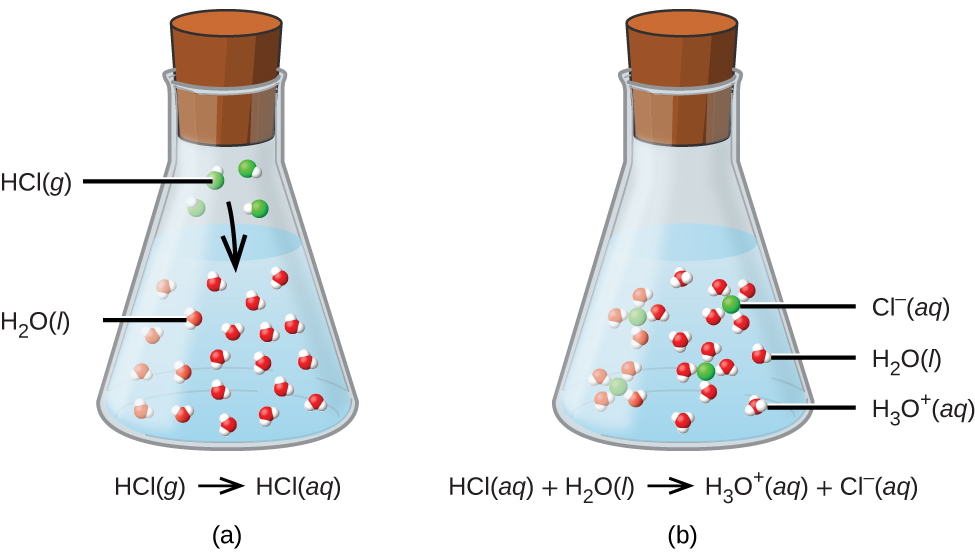
Describe the result of the oxidation of a primary alcohol. Alcohol + sodium → sodium alkoxide + hydrogen. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Water is expelled to generate a carbocation, and the cation. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Describe the result of the oxidation of a secondary alcohol. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. Chemical reactions in alcohols occur mainly at the functional. Thionyl chloride creates an intermediate.
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW
Reaction Between Chlorine And Alcohol A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Describe the result of the oxidation of a primary alcohol. Alcohol + sodium → sodium alkoxide + hydrogen. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Water is expelled to generate a carbocation, and the cation. Chemical reactions in alcohols occur mainly at the functional. Describe the result of the oxidation of a secondary alcohol. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Thionyl chloride creates an intermediate.
From studylib.net
Displacement reactions Reactions of chlorine Reaction Between Chlorine And Alcohol A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Describe the result of the oxidation of a secondary alcohol. Describe the result of the oxidation of a primary alcohol. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. The longer. Reaction Between Chlorine And Alcohol.
From solvedlib.com
Consider the reaction between an alcohol and tosyl ch… SolvedLib Reaction Between Chlorine And Alcohol Thionyl chloride creates an intermediate. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Water is expelled to generate a carbocation, and the cation. Describe the result of the oxidation of a secondary alcohol. Alcohol + sodium → sodium alkoxide + hydrogen. Describe the result of the oxidation. Reaction Between Chlorine And Alcohol.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes Master Organic Chemistry Reaction Between Chlorine And Alcohol A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Water is expelled to generate a carbocation, and the cation. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Thionyl chloride creates an intermediate. Describe the result of the oxidation of. Reaction Between Chlorine And Alcohol.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Reaction Between Chlorine And Alcohol The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Water is expelled to generate a carbocation, and the cation. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. The reaction of a tertiary alcohol with hx takes place by an s n 1. Reaction Between Chlorine And Alcohol.
From www.slideserve.com
PPT Water Treatment Disinfection Processes PowerPoint Presentation Reaction Between Chlorine And Alcohol Chemical reactions in alcohols occur mainly at the functional. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to. Reaction Between Chlorine And Alcohol.
From www.youtube.com
Alkyl Chlorides by Alcohol Reactions with Thionyl Chloride YouTube Reaction Between Chlorine And Alcohol We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Chemical reactions in alcohols occur mainly at the functional. Water is expelled to generate a carbocation, and the cation. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Alcohol + sodium → sodium alkoxide. Reaction Between Chlorine And Alcohol.
From www.youtube.com
Reaction Of Alcohols With Thionyl Chloride Alcohols, Phenols and Reaction Between Chlorine And Alcohol Describe the result of the oxidation of a secondary alcohol. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Water is expelled to generate a carbocation, and the cation. Thionyl chloride creates an intermediate. The reaction of a tertiary alcohol with hx takes place by an s n. Reaction Between Chlorine And Alcohol.
From www.youtube.com
CHEM 222 Reaction of Alcohols with Thionyl Chloride YouTube Reaction Between Chlorine And Alcohol Alcohol + sodium → sodium alkoxide + hydrogen. Chemical reactions in alcohols occur mainly at the functional. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Thionyl chloride creates an intermediate. Describe the result of the oxidation of a primary alcohol. Describe the result of the oxidation of a secondary alcohol. Water is expelled to. Reaction Between Chlorine And Alcohol.
From www.numerade.com
Consider the reaction of acetyl chloride and ethanol (ethyl alcohol) to Reaction Between Chlorine And Alcohol Thionyl chloride creates an intermediate. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. Describe the result of the oxidation of a primary alcohol. Describe the result of the oxidation of a secondary alcohol. A lot of sources on the internet claim that mixing rubbing alcohol. Reaction Between Chlorine And Alcohol.
From www.numerade.com
Consider the reaction between an alcohol and tosyl chloride, followed Reaction Between Chlorine And Alcohol The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. Chemical reactions in alcohols occur mainly at the functional. Water is expelled to generate a carbocation, and the cation. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing. Reaction Between Chlorine And Alcohol.
From chem.libretexts.org
Reactions of Acyl Chlorides with Alcohols Chemistry LibreTexts Reaction Between Chlorine And Alcohol Alcohol + sodium → sodium alkoxide + hydrogen. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Chemical reactions in alcohols occur mainly at the functional. Describe the result of the oxidation. Reaction Between Chlorine And Alcohol.
From www.echemi.com
Reaction of alcohols with PCl5 and PCl3 ECHEMI Reaction Between Chlorine And Alcohol A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Water is expelled to generate a carbocation, and the cation. Describe the result of the oxidation of a primary alcohol. Alcohol + sodium → sodium alkoxide + hydrogen. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Chemical. Reaction Between Chlorine And Alcohol.
From www.onlinemathlearning.com
Organic Chemistry IGCSE Chemistry (solutions, examples, worksheets Reaction Between Chlorine And Alcohol Alcohol + sodium → sodium alkoxide + hydrogen. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Describe the result of the oxidation of a primary alcohol. Thionyl chloride creates an intermediate. Describe the result of the oxidation of a secondary alcohol. The longer the hydrocarbon chain in. Reaction Between Chlorine And Alcohol.
From chem.libretexts.org
10.9 Reactions of Alcohols with Thionyl Chloride Chemistry LibreTexts Reaction Between Chlorine And Alcohol A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Water is expelled to generate a carbocation, and the cation. Describe the result of the oxidation of a secondary alcohol. Thionyl chloride. Reaction Between Chlorine And Alcohol.
From www.youtube.com
Acid Chloride + Alcohol = Ester (Mechanism) YouTube Reaction Between Chlorine And Alcohol The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. Water is expelled to generate a carbocation, and the cation. Describe the result of the oxidation of a primary alcohol. Thionyl chloride creates. Reaction Between Chlorine And Alcohol.
From www.youtube.com
Reaction with Thionyl Chloride YouTube Reaction Between Chlorine And Alcohol A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Describe the result of the oxidation of a primary alcohol. Alcohol + sodium → sodium alkoxide + hydrogen. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Chemical reactions in alcohols. Reaction Between Chlorine And Alcohol.
From www.youtube.com
Chemistry 110, Experiment 15 Part Two Oxidation of Alcohols, Iron Reaction Between Chlorine And Alcohol Thionyl chloride creates an intermediate. Alcohol + sodium → sodium alkoxide + hydrogen. Water is expelled to generate a carbocation, and the cation. Chemical reactions in alcohols occur mainly at the functional. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. A lot of sources on. Reaction Between Chlorine And Alcohol.
From chem.libretexts.org
Acid chlorides react with alcohols to form esters Chemistry LibreTexts Reaction Between Chlorine And Alcohol Alcohol + sodium → sodium alkoxide + hydrogen. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Chemical reactions in alcohols occur mainly at the functional. Water is expelled to generate a carbocation, and the cation. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism. Reaction Between Chlorine And Alcohol.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Reaction Between Chlorine And Alcohol Chemical reactions in alcohols occur mainly at the functional. Thionyl chloride creates an intermediate. Describe the result of the oxidation of a secondary alcohol. Describe the result of the oxidation of a primary alcohol. Alcohol + sodium → sodium alkoxide + hydrogen. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. The. Reaction Between Chlorine And Alcohol.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Reaction Between Chlorine And Alcohol Chemical reactions in alcohols occur mainly at the functional. Alcohol + sodium → sodium alkoxide + hydrogen. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to. Reaction Between Chlorine And Alcohol.
From chem.libretexts.org
10.9 Reactions of Alcohols with Thionyl Chloride Chemistry LibreTexts Reaction Between Chlorine And Alcohol Alcohol + sodium → sodium alkoxide + hydrogen. Describe the result of the oxidation of a secondary alcohol. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Chemical reactions in alcohols occur mainly at. Reaction Between Chlorine And Alcohol.
From solvedlib.com
Consider the reaction between an alcohol and tosyl ch… SolvedLib Reaction Between Chlorine And Alcohol A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Thionyl chloride creates an intermediate. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Water is expelled. Reaction Between Chlorine And Alcohol.
From www.pinterest.jp
Acyl Chlorides with Grignard and Gilman Reagents Reaction Between Chlorine And Alcohol Thionyl chloride creates an intermediate. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. Alcohol + sodium → sodium alkoxide + hydrogen. Describe the result of the oxidation of. Reaction Between Chlorine And Alcohol.
From chem.libretexts.org
Reactions of alcohols with hydrohalic acids (HX) Chemistry LibreTexts Reaction Between Chlorine And Alcohol Describe the result of the oxidation of a secondary alcohol. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. Thionyl chloride creates an intermediate. Alcohol + sodium → sodium alkoxide + hydrogen. Describe the result of the oxidation of a primary alcohol. The longer the hydrocarbon. Reaction Between Chlorine And Alcohol.
From chem.libretexts.org
Acid chlorides react with alcohols to form esters Chemistry LibreTexts Reaction Between Chlorine And Alcohol The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Chemical reactions in alcohols occur mainly at the functional. Alcohol + sodium → sodium alkoxide + hydrogen. Thionyl chloride creates an intermediate. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Describe the result. Reaction Between Chlorine And Alcohol.
From chem.libretexts.org
17.6 Reactions of Alcohols Chemistry LibreTexts Reaction Between Chlorine And Alcohol Thionyl chloride creates an intermediate. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Describe. Reaction Between Chlorine And Alcohol.
From www.chemistrysteps.com
SOCl2 and PBr3 Chemistry Steps Reaction Between Chlorine And Alcohol A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Thionyl chloride creates an intermediate. Chemical reactions in alcohols occur mainly at the functional. The longer the hydrocarbon chain in the alcohol,. Reaction Between Chlorine And Alcohol.
From www.chemistrysteps.com
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps Reaction Between Chlorine And Alcohol A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Describe the result of the oxidation of a primary alcohol. Chemical reactions in alcohols occur mainly at the functional. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. Thionyl. Reaction Between Chlorine And Alcohol.
From www.masterorganicchemistry.com
Reagent Friday Thionyl Chloride (SOCl2) Master Organic Chemistry Reaction Between Chlorine And Alcohol The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Describe the result of the oxidation of a primary alcohol. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Thionyl chloride creates an intermediate. A lot of sources on the internet claim that mixing. Reaction Between Chlorine And Alcohol.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Reaction Between Chlorine And Alcohol Thionyl chloride creates an intermediate. Alcohol + sodium → sodium alkoxide + hydrogen. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. Water is expelled to generate a carbocation,. Reaction Between Chlorine And Alcohol.
From byjus.com
When a chiral [S] alcohol is reacted with SOCl2 in presence of solvent Reaction Between Chlorine And Alcohol Thionyl chloride creates an intermediate. Alcohol + sodium → sodium alkoxide + hydrogen. Describe the result of the oxidation of a secondary alcohol. Water is expelled to generate a carbocation, and the cation. Describe the result of the oxidation of a primary alcohol. Chemical reactions in alcohols occur mainly at the functional. The longer the hydrocarbon chain in the alcohol,. Reaction Between Chlorine And Alcohol.
From www.masterorganicchemistry.com
Making Alkyl Halides From Alcohols Master Organic Chemistry Reaction Between Chlorine And Alcohol Chemical reactions in alcohols occur mainly at the functional. Alcohol + sodium → sodium alkoxide + hydrogen. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. The longer the. Reaction Between Chlorine And Alcohol.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Reaction Between Chlorine And Alcohol Alcohol + sodium → sodium alkoxide + hydrogen. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid protonates the hydroxyl oxygen atom. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Chemical reactions in alcohols occur mainly at the. Reaction Between Chlorine And Alcohol.
From spmchemistry.blog.onlinetuition.com.my
Reaction of Alkali Metals with Chlorine SPM Chemistry Reaction Between Chlorine And Alcohol Describe the result of the oxidation of a primary alcohol. Describe the result of the oxidation of a secondary alcohol. The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. Alcohol + sodium → sodium alkoxide + hydrogen. The reaction of a tertiary alcohol with hx takes place by an s n 1 mechanism when acid. Reaction Between Chlorine And Alcohol.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID5525228 Reaction Between Chlorine And Alcohol The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes. A lot of sources on the internet claim that mixing rubbing alcohol with chlorinated bleach produces chloroform. We have a combination of two reactions, one oxidizing the alcohol to formate ion and the other oxidizing it to chloroform. Describe the result of the oxidation of a. Reaction Between Chlorine And Alcohol.