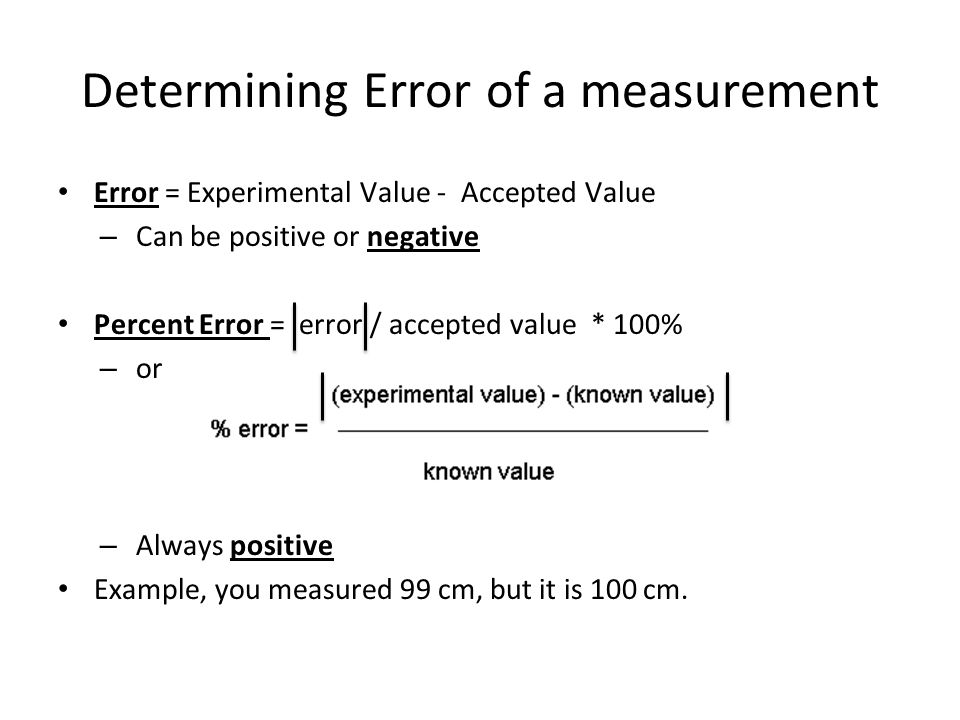
Error In Mass Chemistry . the error of an experiment is the difference between the experimental and accepted values. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. the m/z range for an m/z of interest plus/minus a specified error in ppm. Systematic errors can be caused by faulty. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. when a series of measurements is precise but not accurate, the error is usually systematic. When calculating the theoretical m/z, remember. the error in mass would probably be something to do with how far each measurement deviates from the average. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. This means its mass lies between 6.722 and.
from cristoiublog15.blogspot.com
revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. Systematic errors can be caused by faulty. This means its mass lies between 6.722 and. the error of an experiment is the difference between the experimental and accepted values. the error in mass would probably be something to do with how far each measurement deviates from the average. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. the m/z range for an m/z of interest plus/minus a specified error in ppm. when a series of measurements is precise but not accurate, the error is usually systematic. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or.
Calculate Percent Error Chemistry How to Calculate Percent Error
Error In Mass Chemistry if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. Systematic errors can be caused by faulty. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. When calculating the theoretical m/z, remember. the m/z range for an m/z of interest plus/minus a specified error in ppm. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. the error of an experiment is the difference between the experimental and accepted values. the error in mass would probably be something to do with how far each measurement deviates from the average. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. This means its mass lies between 6.722 and. when a series of measurements is precise but not accurate, the error is usually systematic.
From www.slideserve.com
PPT Introduction to Chemistry & Experimental Error PowerPoint Error In Mass Chemistry — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. when a series of measurements is precise but not accurate, the error is usually. Error In Mass Chemistry.
From www.alpharithms.com
Percent Error Calculator αlphαrithms Error In Mass Chemistry the m/z range for an m/z of interest plus/minus a specified error in ppm. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. This means its mass. Error In Mass Chemistry.
From www.slideserve.com
PPT Nuclear Chemistry PowerPoint Presentation, free download ID6018701 Error In Mass Chemistry the m/z range for an m/z of interest plus/minus a specified error in ppm. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. This means its mass. Error In Mass Chemistry.
From www.tes.com
Measurement calculations/uncertainty for Titrations AS Chemistry Error In Mass Chemistry When calculating the theoretical m/z, remember. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. the error of an experiment is the difference. Error In Mass Chemistry.
From www.tessshebaylo.com
Equation For Percent Error In Chemistry Tessshebaylo Error In Mass Chemistry the m/z range for an m/z of interest plus/minus a specified error in ppm. when a series of measurements is precise but not accurate, the error is usually systematic. When calculating the theoretical m/z, remember. the error in mass would probably be something to do with how far each measurement deviates from the average. revision notes. Error In Mass Chemistry.
From www.slideserve.com
PPT Chapter 5 Errors in Chemical Analysis PowerPoint Presentation Error In Mass Chemistry if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. Systematic errors can be caused by faulty. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. the error of an experiment is the difference between the experimental. Error In Mass Chemistry.
From ar.inspiredpencil.com
Percent Error Formula Chemistry Error In Mass Chemistry to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. the error in mass would probably be something to do with how far each measurement deviates from the average. When calculating the theoretical m/z, remember. when a series of measurements is precise but not accurate, the error. Error In Mass Chemistry.
From guilddelta.weebly.com
How to calculate standard error of the mea guilddelta Error In Mass Chemistry the m/z range for an m/z of interest plus/minus a specified error in ppm. when a series of measurements is precise but not accurate, the error is usually systematic. the error in mass would probably be something to do with how far each measurement deviates from the average. Systematic errors can be caused by faulty. the. Error In Mass Chemistry.
From sciencenotes.org
Calculate Percent Error Error In Mass Chemistry — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. when a series of measurements is precise but not accurate, the error is usually systematic. When calculating the theoretical m/z, remember. the m/z range for an m/z of interest plus/minus a specified error in. Error In Mass Chemistry.
From howtohacks92.blogspot.com
Calculate Percent Error Chemistry Equation For Percent Change In Mass Error In Mass Chemistry When calculating the theoretical m/z, remember. This means its mass lies between 6.722 and. Systematic errors can be caused by faulty. the error in mass would probably be something to do with how far each measurement deviates from the average. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry. Error In Mass Chemistry.
From www.slideshare.net
1 measurement and error Error In Mass Chemistry to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. the error in mass would probably be something to do with how far each. Error In Mass Chemistry.
From www.youtube.com
Introduction to Error Analysis for Chemistry Lab YouTube Error In Mass Chemistry if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. This means its mass lies between 6.722 and. the error of an experiment is the difference between the experimental and accepted values. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value. Error In Mass Chemistry.
From locallopi.weebly.com
Percent error chemistry calculator locallopi Error In Mass Chemistry revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. the error of an experiment is the difference between the experimental. Error In Mass Chemistry.
From learningzonestrauss.z19.web.core.windows.net
Percent Error Worksheet Answer Key Error In Mass Chemistry revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. the error of an experiment is the difference between the experimental and accepted values. the error in mass would probably be something to do with how far each measurement deviates from the average. . Error In Mass Chemistry.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors Error In Mass Chemistry When calculating the theoretical m/z, remember. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. the error in mass would probably be something to do with how far each measurement deviates from the average. the m/z range for an m/z of interest plus/minus a specified error. Error In Mass Chemistry.
From www.askiitians.com
Accuracy, Precision of Instruments and Errors in Measurements Study Error In Mass Chemistry the error of an experiment is the difference between the experimental and accepted values. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. the error in mass would probably be something to do with how far each measurement deviates from the average. . Error In Mass Chemistry.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors Error In Mass Chemistry if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. This means its mass lies between 6.722 and. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. the error in mass would probably be something to do with how far. Error In Mass Chemistry.
From www.youtube.com
The percentage errors in the measurement of mass and speed are `2 and Error In Mass Chemistry revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. When calculating the theoretical m/z, remember. Systematic errors can be caused by faulty. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known. Error In Mass Chemistry.
From thechemistrynotes.com
Absolute and Relative Error Definition, Formula, Examples, Differences Error In Mass Chemistry when a series of measurements is precise but not accurate, the error is usually systematic. Systematic errors can be caused by faulty. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts. Error In Mass Chemistry.
From www.showme.com
Scientific Notation and Percent Error Science, Chemistry ShowMe Error In Mass Chemistry when a series of measurements is precise but not accurate, the error is usually systematic. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. the error of an experiment is the difference between the experimental and accepted values. When calculating the theoretical m/z, remember. This means its mass lies between. Error In Mass Chemistry.
From howtohacks92.blogspot.com
Calculate Percent Error Chemistry Equation For Percent Change In Mass Error In Mass Chemistry if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as. Error In Mass Chemistry.
From dxodaldge.blob.core.windows.net
Definition Of Error In Chemistry at Natasha McKnight blog Error In Mass Chemistry when a series of measurements is precise but not accurate, the error is usually systematic. When calculating the theoretical m/z, remember. Systematic errors can be caused by faulty. the error in mass would probably be something to do with how far each measurement deviates from the average. This means its mass lies between 6.722 and. the m/z. Error In Mass Chemistry.
From howtohacks92.blogspot.com
Calculate Percent Error Chemistry Equation For Percent Change In Mass Error In Mass Chemistry the error of an experiment is the difference between the experimental and accepted values. This means its mass lies between 6.722 and. When calculating the theoretical m/z, remember. when a series of measurements is precise but not accurate, the error is usually systematic. — percent error, sometimes referred to as percentage error, is an expression of the. Error In Mass Chemistry.
From www.expii.com
Mass Defect — Definition & Overview Expii Error In Mass Chemistry if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. This means its mass lies between 6.722 and. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. when a series of measurements is precise but not accurate,. Error In Mass Chemistry.
From ar.inspiredpencil.com
Percent Error Formula Chemistry Error In Mass Chemistry Systematic errors can be caused by faulty. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. when a series of measurements is precise but not accurate, the error is usually systematic. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value. Error In Mass Chemistry.
From cristoiublog15.blogspot.com
Calculate Percent Error Chemistry How to Calculate Percent Error Error In Mass Chemistry This means its mass lies between 6.722 and. the error in mass would probably be something to do with how far each measurement deviates from the average. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus,. Error In Mass Chemistry.
From www.youtube.com
Percent Error Calculations in Chemistry Regents Chemistry YouTube Error In Mass Chemistry — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. This means its mass lies between 6.722 and. to illustrate how the number of significant digits indicates the. Error In Mass Chemistry.
From www.slideshare.net
Errors in chemical analysis Error In Mass Chemistry the m/z range for an m/z of interest plus/minus a specified error in ppm. when a series of measurements is precise but not accurate, the error is usually systematic. When calculating the theoretical m/z, remember. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known. Error In Mass Chemistry.
From schematicfixshapings.z19.web.core.windows.net
How To Calculate Error Rate Error In Mass Chemistry the m/z range for an m/z of interest plus/minus a specified error in ppm. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. When calculating the theoretical. Error In Mass Chemistry.
From www.youtube.com
Spotting procedural errors in chemistry practicals YouTube Error In Mass Chemistry the error in mass would probably be something to do with how far each measurement deviates from the average. if the coin is weighed on a more sensitive balance, the mass might be 6.723 g. This means its mass lies between 6.722 and. When calculating the theoretical m/z, remember. to illustrate how the number of significant digits. Error In Mass Chemistry.
From ar.inspiredpencil.com
Absolute Error Formula Chemistry Error In Mass Chemistry This means its mass lies between 6.722 and. When calculating the theoretical m/z, remember. the m/z range for an m/z of interest plus/minus a specified error in ppm. the error in mass would probably be something to do with how far each measurement deviates from the average. the error of an experiment is the difference between the. Error In Mass Chemistry.
From www.showme.com
Percent Error chemistry Science, Chemistry, Measurements and Error In Mass Chemistry the m/z range for an m/z of interest plus/minus a specified error in ppm. This means its mass lies between 6.722 and. when a series of measurements is precise but not accurate, the error is usually systematic. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and. Error In Mass Chemistry.
From cristoiublog15.blogspot.com
Calculate Percent Error Chemistry How to Calculate Percent Error Error In Mass Chemistry When calculating the theoretical m/z, remember. revision notes on 1.7.3 error & uncertainty for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. — percent error, sometimes referred to as percentage error, is an expression of the difference between a measured value and the known or. the error of an experiment. Error In Mass Chemistry.
From www.showme.com
Percent error part 2 Chemistry, Science, Stoichiometry ShowMe Error In Mass Chemistry when a series of measurements is precise but not accurate, the error is usually systematic. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g. the m/z range for an m/z of interest plus/minus a specified error in ppm. if the coin is weighed on a. Error In Mass Chemistry.
From www.slideserve.com
PPT Uncertainty & Errors in Measurement PowerPoint Presentation ID Error In Mass Chemistry the m/z range for an m/z of interest plus/minus a specified error in ppm. This means its mass lies between 6.722 and. when a series of measurements is precise but not accurate, the error is usually systematic. to illustrate how the number of significant digits indicates the error, suppose we had a measurement reported as 3.42 g.. Error In Mass Chemistry.