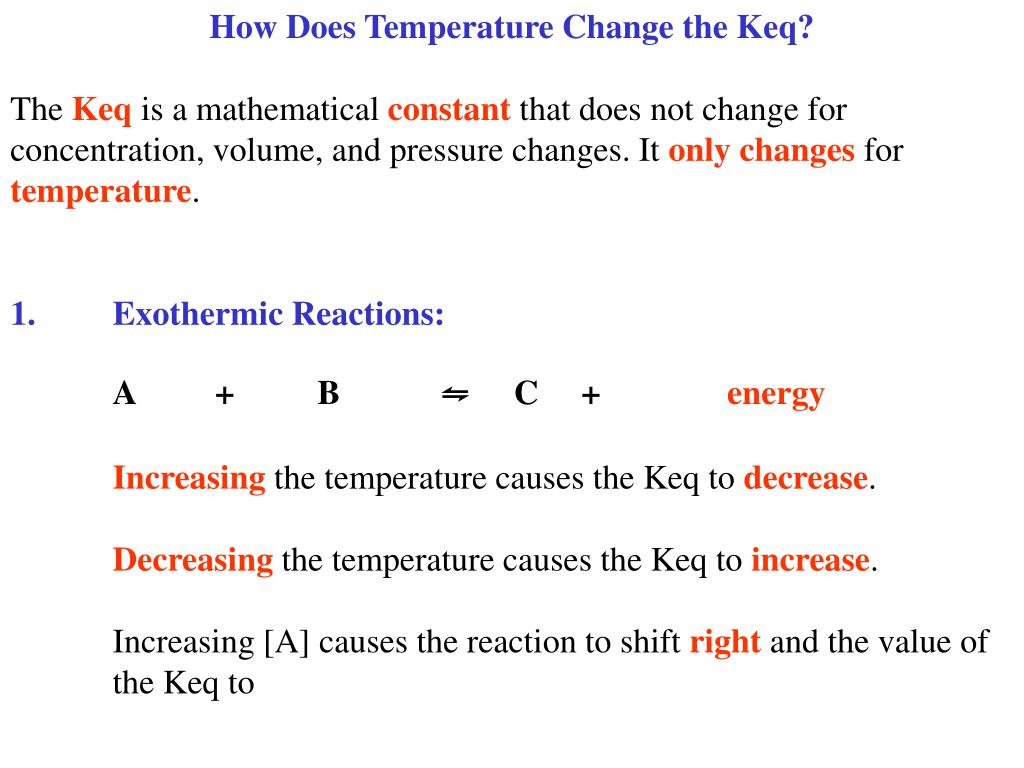
Does Temperature Affect Keq . For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. the k eq decreases in value and heat is added to an exothermic reaction. when temperature is the stress that affects a system at equilibrium, there are two important consequences: the k eq is a characteristic numerical value for a given reaction at a given temperature; Keq k eq will decrease as the temperature increases. equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. the simplest interpretation is as follows: the calculation of k at a particular temperature from a known k at another given temperature can be approached as follows if standard thermodynamic. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium.
from www.slideserve.com
the simplest interpretation is as follows: equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. the k eq is a characteristic numerical value for a given reaction at a given temperature; the k eq decreases in value and heat is added to an exothermic reaction. N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): Keq k eq will decrease as the temperature increases. the calculation of k at a particular temperature from a known k at another given temperature can be approached as follows if standard thermodynamic. when temperature is the stress that affects a system at equilibrium, there are two important consequences:
PPT Temperature and Keq Lesson 11 PowerPoint Presentation, free
Does Temperature Affect Keq when temperature is the stress that affects a system at equilibrium, there are two important consequences: the k eq decreases in value and heat is added to an exothermic reaction. N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): the calculation of k at a particular temperature from a known k at another given temperature can be approached as follows if standard thermodynamic. when temperature is the stress that affects a system at equilibrium, there are two important consequences: the simplest interpretation is as follows: Keq k eq will decrease as the temperature increases. the k eq is a characteristic numerical value for a given reaction at a given temperature;
From www.slideserve.com
PPT Temperature and Keq Lesson 11 PowerPoint Presentation, free Does Temperature Affect Keq the k eq decreases in value and heat is added to an exothermic reaction. N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. the calculation of k at a particular temperature from a known k at another given temperature can be approached as follows if standard thermodynamic. the k eq is a characteristic numerical. Does Temperature Affect Keq.
From www.reddit.com
Why does Keq change with temperature? r/Mcat Does Temperature Affect Keq Keq k eq will decrease as the temperature increases. the k eq decreases in value and heat is added to an exothermic reaction. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. N 2 o 4 (g) + heat. Does Temperature Affect Keq.
From www.toppr.com
A schematic plot of Keq. versus inverse of temperature for a reaction Does Temperature Affect Keq when temperature is the stress that affects a system at equilibrium, there are two important consequences: the k eq decreases in value and heat is added to an exothermic reaction. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. the calculation of k at a particular temperature from a known k. Does Temperature Affect Keq.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does Temperature Affect Keq Keq k eq will decrease as the temperature increases. the k eq decreases in value and heat is added to an exothermic reaction. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. the simplest interpretation is as follows: when. Does Temperature Affect Keq.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Does Temperature Affect Keq the k eq is a characteristic numerical value for a given reaction at a given temperature; equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): Equilibrium constants aren't changed if you change the concentrations of things present in the. Does Temperature Affect Keq.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does Temperature Affect Keq Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. the k eq is a characteristic numerical value for a given reaction at a given temperature; Keq k eq will decrease as the temperature increases. the simplest interpretation is as follows: the k eq decreases in value and heat is added to. Does Temperature Affect Keq.
From kunduz.com
[ANSWERED] A schematic plot of In Keq versus inverse of temperature for Does Temperature Affect Keq equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. when temperature is the stress that affects a system at equilibrium, there are two important consequences: the simplest interpretation is as follows: the k eq is a characteristic numerical value for a given reaction at a given temperature; the. Does Temperature Affect Keq.
From www.slideserve.com
PPT SECTION 1 THE CONCEPT OF EQUILIBRIUM PowerPoint Presentation Does Temperature Affect Keq Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. when temperature is the stress that affects a system at equilibrium, there are two important consequences: the k eq is a characteristic numerical value for a given reaction at a given temperature; equilibrium constants (keq) are a way of the quotient of. Does Temperature Affect Keq.
From slidetodoc.com
11 3 Le Chateliers Principle Shifts in Equilibrium Does Temperature Affect Keq the simplest interpretation is as follows: the calculation of k at a particular temperature from a known k at another given temperature can be approached as follows if standard thermodynamic. the k eq is a characteristic numerical value for a given reaction at a given temperature; Keq k eq will decrease as the temperature increases. For an. Does Temperature Affect Keq.
From slideplayer.com
N2O4(g) + energy 2 NO2(g) (colourless) (reddishbrown) ppt download Does Temperature Affect Keq the k eq decreases in value and heat is added to an exothermic reaction. N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. the k eq is a characteristic numerical value for a given reaction at a given temperature; equilibrium constants (keq) are a way of the quotient of a reaction which has reached. Does Temperature Affect Keq.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does Temperature Affect Keq the calculation of k at a particular temperature from a known k at another given temperature can be approached as follows if standard thermodynamic. equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. the k eq decreases in value and heat is added to an exothermic reaction. the k. Does Temperature Affect Keq.
From www.researchgate.net
Results for Keq as function of pressure and temperature difference Does Temperature Affect Keq the simplest interpretation is as follows: equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. Keq k eq will decrease as the temperature increases. the k eq is a characteristic numerical value for a given reaction at a given temperature; the calculation of k at a particular temperature from. Does Temperature Affect Keq.
From slideplayer.com
Equilibrium & ppt download Does Temperature Affect Keq For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): the k eq is a characteristic numerical value for a given reaction at a given temperature; the k eq decreases in value and heat is added to an exothermic reaction. the calculation of k at a particular temperature from a known k at another given. Does Temperature Affect Keq.
From www.slideserve.com
PPT Equilibrium Calculations PowerPoint Presentation, free download Does Temperature Affect Keq N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. when temperature is the stress that affects a system at equilibrium, there are two important consequences: the k eq decreases in value and heat is added to an exothermic reaction. the calculation of k at a particular temperature from a known k at another given. Does Temperature Affect Keq.
From www.slideserve.com
PPT Temperature and Keq Lesson 11 PowerPoint Presentation, free Does Temperature Affect Keq the simplest interpretation is as follows: the k eq is a characteristic numerical value for a given reaction at a given temperature; N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. Keq k eq will decrease as the temperature increases. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): equilibrium constants. Does Temperature Affect Keq.
From www.slideserve.com
PPT HUKUM KEDUA TERMODINAMIKA PowerPoint Presentation, free download Does Temperature Affect Keq the k eq is a characteristic numerical value for a given reaction at a given temperature; equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. the simplest interpretation is as follows: when temperature is the stress that affects a system at equilibrium, there are two important consequences: Equilibrium constants. Does Temperature Affect Keq.
From slideplayer.com
Chapter 17 Thermodynamics Spontaneity, Entropy, and Free Energy ppt Does Temperature Affect Keq the k eq decreases in value and heat is added to an exothermic reaction. Keq k eq will decrease as the temperature increases. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. the calculation of. Does Temperature Affect Keq.
From slideplayer.com
DO NOW Pick up notes. Turn in Concentration and Reaction Rate lab Does Temperature Affect Keq equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. Keq k eq will decrease as the temperature increases. N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. the k eq is a characteristic numerical value for a given reaction at a given temperature; For an exothermic reaction (δh−⊖−. Does Temperature Affect Keq.
From slideplayer.com
Chem 10 Equilibrium Chp 15 Spr ppt download Does Temperature Affect Keq when temperature is the stress that affects a system at equilibrium, there are two important consequences: For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): Keq k eq will decrease as the temperature increases. the k eq is a characteristic numerical value for a given reaction at a given temperature; N 2 o 4 (g). Does Temperature Affect Keq.
From slideplayer.com
Chemical Equilibrium Forward and reverse reactions taking place at Does Temperature Affect Keq Keq k eq will decrease as the temperature increases. the simplest interpretation is as follows: the k eq decreases in value and heat is added to an exothermic reaction. equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. the k eq is a characteristic numerical value for a given. Does Temperature Affect Keq.
From www.toppr.com
3. A schematic plot of In Keq VS inverse of temperature a reaction is Does Temperature Affect Keq For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. the k eq. Does Temperature Affect Keq.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271575 Does Temperature Affect Keq the simplest interpretation is as follows: the calculation of k at a particular temperature from a known k at another given temperature can be approached as follows if standard thermodynamic. N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. when temperature is the stress that affects a system at equilibrium, there are two important. Does Temperature Affect Keq.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does Temperature Affect Keq the k eq decreases in value and heat is added to an exothermic reaction. equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. when temperature is the stress that affects a system at equilibrium, there. Does Temperature Affect Keq.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does Temperature Affect Keq the k eq decreases in value and heat is added to an exothermic reaction. Keq k eq will decrease as the temperature increases. when temperature is the stress that affects a system at equilibrium, there are two important consequences: equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. the. Does Temperature Affect Keq.
From www.slideserve.com
PPT VI. The Equilibrium Constant PowerPoint Presentation, free Does Temperature Affect Keq the k eq decreases in value and heat is added to an exothermic reaction. Keq k eq will decrease as the temperature increases. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): when temperature is the stress that affects a system at equilibrium, there are two important consequences: N 2 o 4 (g) + heat. Does Temperature Affect Keq.
From www.slideserve.com
PPT Temperature and Keq Lesson 11 PowerPoint Presentation, free Does Temperature Affect Keq N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. the k eq decreases in value and heat is added to an exothermic reaction. the simplest interpretation is as follows: Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. the calculation of k at a particular temperature from a. Does Temperature Affect Keq.
From www.numerade.com
SOLVEDHow does decreasing the temperature affect the value of Ke q for Does Temperature Affect Keq N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): the k eq decreases in value and heat is added to an exothermic reaction. the simplest interpretation is as follows: the calculation of k at a particular temperature from a known k at. Does Temperature Affect Keq.
From slideplayer.com
Equilibrium Constants ppt download Does Temperature Affect Keq the k eq decreases in value and heat is added to an exothermic reaction. the simplest interpretation is as follows: Keq k eq will decrease as the temperature increases. equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. when temperature is the stress that affects a system at equilibrium,. Does Temperature Affect Keq.
From www.slideserve.com
PPT Temperature and Keq Lesson 11 PowerPoint Presentation, free Does Temperature Affect Keq For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. the k eq is a characteristic numerical value for a given reaction at a given temperature; Keq k eq will decrease as the temperature increases. the k eq decreases in value and heat is. Does Temperature Affect Keq.
From slideplayer.com
Equilibrium L. ppt download Does Temperature Affect Keq Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): the k eq decreases in value and heat is added to an exothermic reaction. Keq. Does Temperature Affect Keq.
From www.slideserve.com
PPT Review Expressions of the thermodynamic equilibrium constant K Does Temperature Affect Keq the k eq is a characteristic numerical value for a given reaction at a given temperature; when temperature is the stress that affects a system at equilibrium, there are two important consequences: Keq k eq will decrease as the temperature increases. the calculation of k at a particular temperature from a known k at another given temperature. Does Temperature Affect Keq.
From www.slideserve.com
PPT Equilibrium Rate of Reaction Understanding Dynamic Equilibrium Does Temperature Affect Keq when temperature is the stress that affects a system at equilibrium, there are two important consequences: equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. N 2 o 4 (g) + heat ⇌ 2no 2 (g) which. Keq k eq will decrease as the temperature increases. the simplest interpretation is. Does Temperature Affect Keq.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Does Temperature Affect Keq For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. Keq k eq will decrease as the temperature increases. the k eq decreases in value and heat is added to an exothermic reaction. when temperature is the stress that affects a. Does Temperature Affect Keq.
From www.tessshebaylo.com
Equilibrium Temperature Equation Tessshebaylo Does Temperature Affect Keq the k eq decreases in value and heat is added to an exothermic reaction. For an exothermic reaction (δh−⊖− <0 δ h − ⊖ − <0): Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. the simplest interpretation is as follows: the k eq is a characteristic numerical value for a. Does Temperature Affect Keq.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Does Temperature Affect Keq when temperature is the stress that affects a system at equilibrium, there are two important consequences: Keq k eq will decrease as the temperature increases. the simplest interpretation is as follows: equilibrium constants (keq) are a way of the quotient of a reaction which has reached equilibrium. Equilibrium constants aren't changed if you change the concentrations of. Does Temperature Affect Keq.