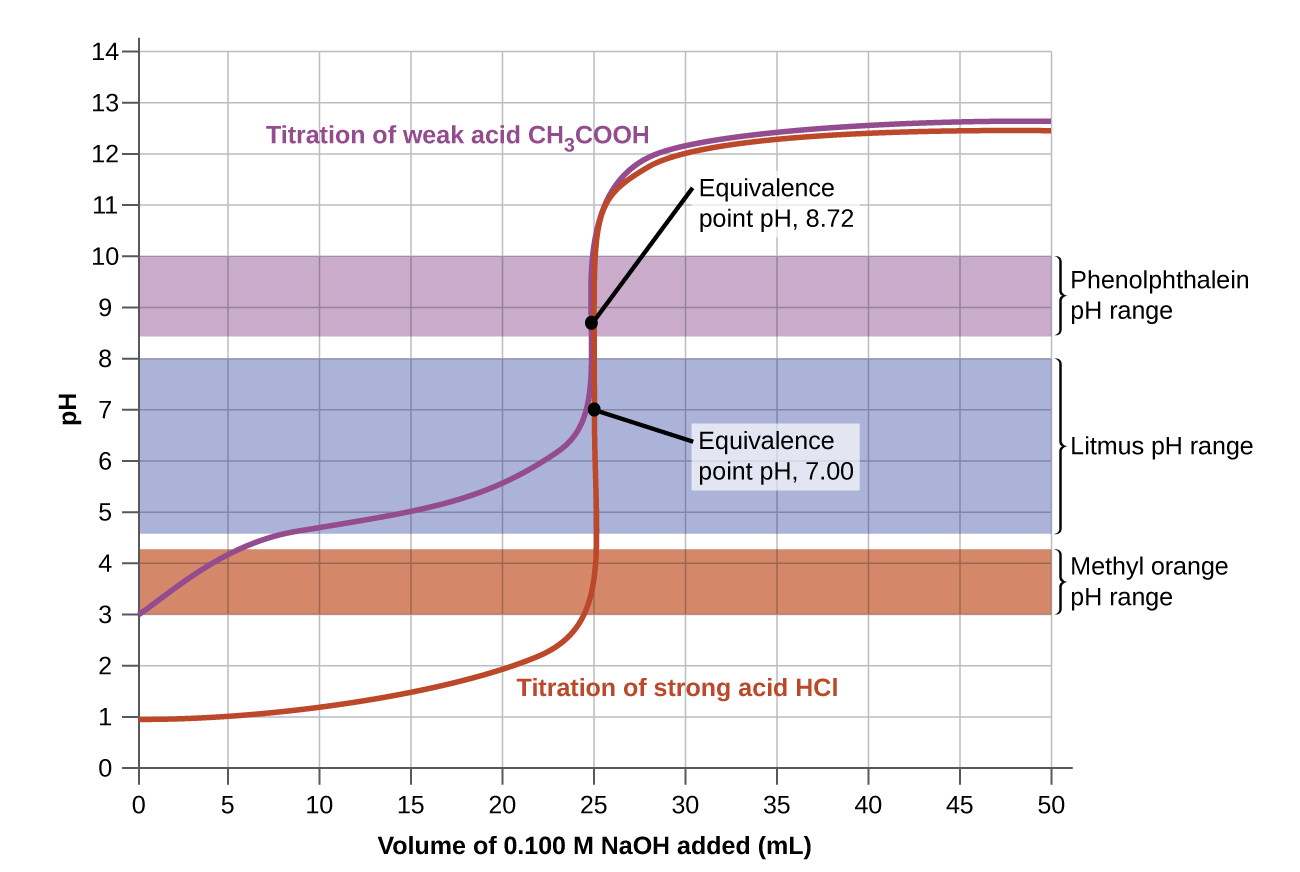
Indicator Titrations Definition . An indicator reacts with an. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In many titrations, you use a. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. They are usually weak acids or. indicators are substances whose solutions change color due to changes in ph.
from fyobaweqw.blob.core.windows.net
In many titrations, you use a. an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states. They are usually weak acids or. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. An indicator reacts with an. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. indicators are substances whose solutions change color due to changes in ph. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
Indicators Titration Define at James Honeycutt blog
Indicator Titrations Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. They are usually weak acids or. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. indicators are substances whose solutions change color due to changes in ph. In many titrations, you use a. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. An indicator reacts with an. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.
From hxedyezjh.blob.core.windows.net
Acid Base Indicators Titration at Julie Corson blog Indicator Titrations Definition They are usually weak acids or. indicators are substances whose solutions change color due to changes in ph. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of. Indicator Titrations Definition.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Indicator Titrations Definition They are usually weak acids or. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. An indicator reacts with an. an indicator is. Indicator Titrations Definition.
From giohqmnus.blob.core.windows.net
Titration Definition Wikipedia at James Henrich blog Indicator Titrations Definition In many titrations, you use a. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. indicators are substances whose solutions change color due to changes in ph. an indicator is a weak acid or a weak base that has different colors in its. Indicator Titrations Definition.
From hxenozygz.blob.core.windows.net
Titration Definition And Indicator at Karen Pogue blog Indicator Titrations Definition in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. indicators are substances whose solutions change color due to. Indicator Titrations Definition.
From www.studypool.com
SOLUTION Acid base indicators titration curves Studypool Indicator Titrations Definition An indicator reacts with an. They are usually weak acids or. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. In many titrations,. Indicator Titrations Definition.
From mungfali.com
Acid Base Titration Indicator Indicator Titrations Definition In many titrations, you use a. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. An indicator reacts with an. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. an indicator is a. Indicator Titrations Definition.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Indicator Titrations Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. indicators are substances whose solutions change color due to changes in ph. an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states. titration is the. Indicator Titrations Definition.
From teachntest.org
Precipitation Titration Definition, Principle Indicators, Curve Indicator Titrations Definition They are usually weak acids or. indicators are substances whose solutions change color due to changes in ph. an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding. Indicator Titrations Definition.
From www.studypool.com
SOLUTION Acid base indicators titration curves Studypool Indicator Titrations Definition In many titrations, you use a. An indicator reacts with an. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. chemical indicator, any substance that gives. Indicator Titrations Definition.
From hxeijsdbi.blob.core.windows.net
Titrimetric Analysis Definition at Jessie Winningham blog Indicator Titrations Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. They are usually weak acids or. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. titration is the slow addition of one. Indicator Titrations Definition.
From thechemistrynotes.com
Precipitation Titration Definition, Indicators, Advantages, Indicator Titrations Definition An indicator reacts with an. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. They are usually weak acids or. indicators are substances whose solutions change color due to changes in ph. an indicator is a weak acid or a weak base that has different. Indicator Titrations Definition.
From fyobaweqw.blob.core.windows.net
Indicators Titration Define at James Honeycutt blog Indicator Titrations Definition chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. In many titrations, you use a. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. They are usually weak acids or.. Indicator Titrations Definition.
From scienceinfo.com
Precipitation Titration Definition, Indicators, Advantages, Indicator Titrations Definition An indicator reacts with an. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. In many titrations, you use a. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. . Indicator Titrations Definition.
From gioteyspe.blob.core.windows.net
Indicator Used For Complexometric Titrations at Jerlene Powell blog Indicator Titrations Definition An indicator reacts with an. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration, process of chemical analysis in which the quantity of some constituent of. Indicator Titrations Definition.
From www.youtube.com
Titration Definition, steps and formula YouTube Indicator Titrations Definition in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or. an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states.. Indicator Titrations Definition.
From giokbmeof.blob.core.windows.net
Base Indicator In Titration at Pam Granville blog Indicator Titrations Definition a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In many titrations, you use a. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. indicators are substances whose solutions change color due to changes in ph.. Indicator Titrations Definition.
From fyobaweqw.blob.core.windows.net
Indicators Titration Define at James Honeycutt blog Indicator Titrations Definition They are usually weak acids or. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. indicators are substances whose solutions change color due to changes in ph. In many titrations, you use a. titration, process of chemical analysis in which the quantity of. Indicator Titrations Definition.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Indicator Titrations Definition in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. An indicator reacts with an. In many titrations, you use a. They are usually weak acids or. an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states. a. Indicator Titrations Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator Titrations Definition indicators are substances whose solutions change color due to changes in ph. An indicator reacts with an. In many titrations, you use a. They are usually weak acids or. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. an indicator is a weak acid or a weak. Indicator Titrations Definition.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Indicator Titrations Definition An indicator reacts with an. indicators are substances whose solutions change color due to changes in ph. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. In many titrations, you use a. an indicator is a weak acid or a weak base that. Indicator Titrations Definition.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Indicator Titrations Definition an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states. indicators are substances whose solutions change color due to changes in ph. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In many titrations, you use. Indicator Titrations Definition.
From gioxhprmf.blob.core.windows.net
Titration Significant Figures at Darlene Maloy blog Indicator Titrations Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. An indicator reacts with an. They are usually weak acids or. in a. Indicator Titrations Definition.
From hxelcilfq.blob.core.windows.net
What Is Titration In Chemistry Easy Definition at Kayla Walters blog Indicator Titrations Definition They are usually weak acids or. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In many titrations, you use a. indicators are substances whose solutions change color due to changes in ph. chemical indicator, any substance that gives a visible sign, usually by a color. Indicator Titrations Definition.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Indicator Titrations Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration, process of chemical analysis in which the quantity of some constituent of a. Indicator Titrations Definition.
From fyoxdsbeu.blob.core.windows.net
Titration Experiment Indicator at Shirley Swan blog Indicator Titrations Definition They are usually weak acids or. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a. indicators are substances whose solutions change color due to changes in ph. An indicator reacts with an. chemical indicator, any substance that gives a visible sign,. Indicator Titrations Definition.
From fyobaweqw.blob.core.windows.net
Indicators Titration Define at James Honeycutt blog Indicator Titrations Definition In many titrations, you use a. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. They are usually weak acids or. An indicator reacts with. Indicator Titrations Definition.
From collegedunia.com
Titration Formula Types, Process & Acid Base Indicators Indicator Titrations Definition in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. They are usually weak acids or. an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states. titration is the slow addition of one solution of a known concentration. Indicator Titrations Definition.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID2134619 Indicator Titrations Definition An indicator reacts with an. in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a. chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. a titration is a. Indicator Titrations Definition.
From fyobaweqw.blob.core.windows.net
Indicators Titration Define at James Honeycutt blog Indicator Titrations Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. indicators are substances whose solutions change color due to changes in ph. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. They. Indicator Titrations Definition.
From exyxqrdzm.blob.core.windows.net
Chemistry Titration Indicators at Richard Veit blog Indicator Titrations Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. They are usually weak acids or. chemical indicator, any substance that gives a visible sign, usually. Indicator Titrations Definition.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator Titrations Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. an indicator is a weak acid or a weak base that has different colors in its dissociated and undissociated states. In many titrations, you use a. a titration is a laboratory technique used to. Indicator Titrations Definition.
From giofzfwwv.blob.core.windows.net
Acid Base Titration To Determine The Concentration at Latonya John blog Indicator Titrations Definition a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. indicators are substances whose solutions change color due to changes in ph. an. Indicator Titrations Definition.
From worksheetcampusslewed.z21.web.core.windows.net
How To Do Titrations In Chemistry Indicator Titrations Definition a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In many titrations, you use a. They are usually weak acids or. An indicator reacts with an. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the.. Indicator Titrations Definition.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Indicator Titrations Definition chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold concentration. In many titrations, you use a. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. They are usually weak acids or. an. Indicator Titrations Definition.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Indicator Titrations Definition in a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. An indicator reacts with an. indicators are substances whose solutions change color due to changes in ph. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Indicator Titrations Definition.