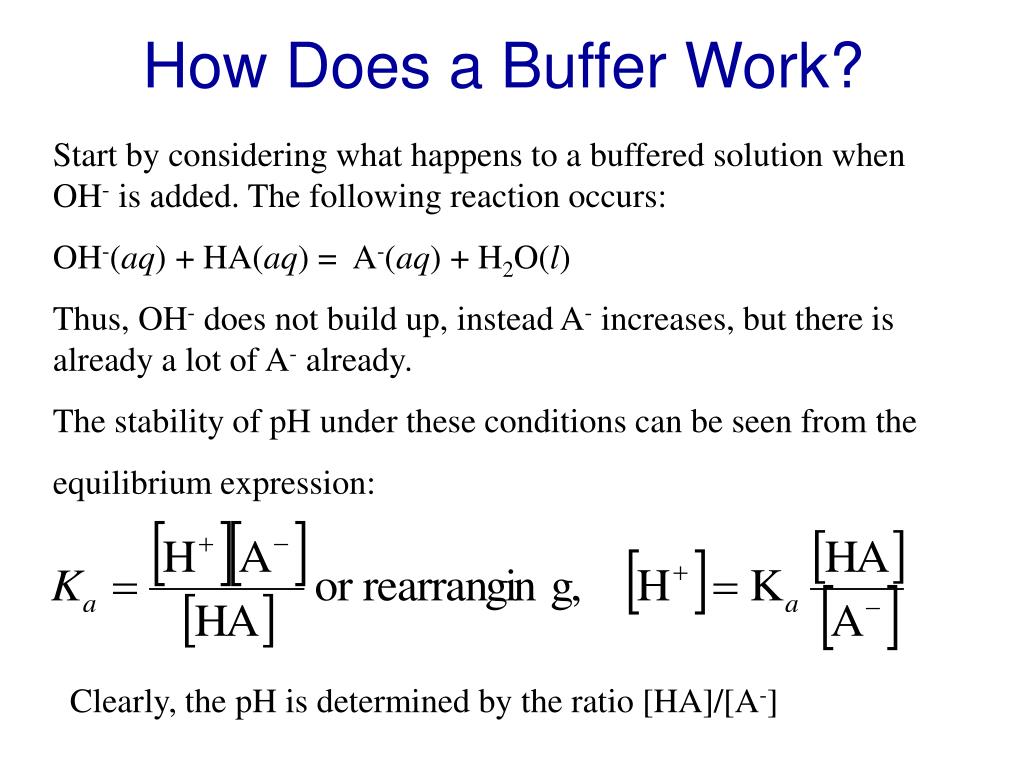
What Is A Buffer Value . a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. If the ph of a buffer goes out of this range, the buffer will no. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.
from www.slideserve.com
a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. If the ph of a buffer goes out of this range, the buffer will no. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
PPT All About Buffers PowerPoint Presentation, free download ID1321295
What Is A Buffer Value If the ph of a buffer goes out of this range, the buffer will no. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. If the ph of a buffer goes out of this range, the buffer will no. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Value a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a mixture of a weak acid and its conjugate base (or a mixture of. What Is A Buffer Value.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Is A Buffer Value a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. a mixture of a weak acid and its conjugate base (or. What Is A Buffer Value.
From www.slideserve.com
PPT All About Buffers PowerPoint Presentation, free download ID1321295 What Is A Buffer Value a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. If the ph of a buffer goes out of this range, the buffer will no. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. Buffers are. What Is A Buffer Value.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID What Is A Buffer Value a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. a buffer is a solution that can resist ph change upon the addition of an. What Is A Buffer Value.
From www.slideserve.com
PPT Weak acids and bases Salt of weak acid and bases buffer Lecture 9 What Is A Buffer Value a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. If the ph of a buffer goes out of this range, the buffer will no. Buffers are. What Is A Buffer Value.
From exyuokede.blob.core.windows.net
Titration Graph Buffer at Mark Paras blog What Is A Buffer Value a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by the ph range over which they can maintain. What Is A Buffer Value.
From courses.lumenlearning.com
Buffers Chemistry What Is A Buffer Value a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. a buffer solution is a solution where the ph does not. What Is A Buffer Value.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free What Is A Buffer Value a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. If the ph of a buffer goes out of this range, the. What Is A Buffer Value.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube What Is A Buffer Value a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that maintains the stability of a system’s ph level. What Is A Buffer Value.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is A Buffer Value If the ph of a buffer goes out of this range, the buffer will no. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or. What Is A Buffer Value.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is A Buffer Value a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are characterized by the ph range over which they can maintain a more or less constant. What Is A Buffer Value.
From www.slideshare.net
Buffer system What Is A Buffer Value a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer solution is a solution where the ph does not. What Is A Buffer Value.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is A Buffer Value a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. a solution whose ph is not altered to any great extent by the. What Is A Buffer Value.
From buffer.com
The 10 Buffer Values and How We Act on Them Every Day What Is A Buffer Value a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and. What Is A Buffer Value.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation What Is A Buffer Value a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. If the ph of a buffer goes out of this range, the buffer will no. a buffer has an effective ph range of one ph unit on either side of the pkₐ value. What Is A Buffer Value.
From www.researchgate.net
6. Schematic diagram of the buffer capacity of an aqueous system What Is A Buffer Value a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a solution whose ph is not altered to any great extent by the addition of small. What Is A Buffer Value.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation What Is A Buffer Value a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. a solution whose ph is not altered to any great extent. What Is A Buffer Value.
From www.youtube.com
What is Buffer ? Why Buffer and TriState Buffers are used in Digital What Is A Buffer Value a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. a buffer solution is a solution where the ph does not change significantly on dilution or. What Is A Buffer Value.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts What Is A Buffer Value a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that can. What Is A Buffer Value.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Buffer Value a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. a mixture of a weak acid and its conjugate base (or a mixture of a. What Is A Buffer Value.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Buffer Value If the ph of a buffer goes out of this range, the buffer will no. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for. What Is A Buffer Value.
From www.youtube.com
Buffer range and capacity YouTube What Is A Buffer Value a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. If the ph of a buffer goes out of this range, the buffer will no. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. What Is A Buffer Value.
From open.buffer.com
The 10 Buffer Values and How We Act on Them Every Day What Is A Buffer Value If the ph of a buffer goes out of this range, the buffer will no. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called. What Is A Buffer Value.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint What Is A Buffer Value a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. a buffer solution is a solution where the ph. What Is A Buffer Value.
From www.youtube.com
Buffer capacity; making a buffer with a given pH value YouTube What Is A Buffer Value a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. Buffers are characterized by the ph range over. What Is A Buffer Value.
From golifescience.com
Buffer Solution definition, 4 Types and Basic Calculations What Is A Buffer Value a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. a buffer solution is a solution where the ph. What Is A Buffer Value.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination for Biologists What Is A Buffer Value a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. If the ph of a buffer goes out of this range, the buffer will no. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its. What Is A Buffer Value.
From www.youtube.com
Buffers and Titration Curves YouTube What Is A Buffer Value a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. a buffer is a solution that can resist ph. What Is A Buffer Value.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Is A Buffer Value a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. If the ph of a buffer goes out. What Is A Buffer Value.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions What Is A Buffer Value a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid. a mixture of a weak acid and its conjugate base. What Is A Buffer Value.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses What Is A Buffer Value a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. a buffer has an effective ph range of one ph unit on. What Is A Buffer Value.
From courses.lumenlearning.com
pH, Buffers, Acids, and Bases Introduction to Chemistry What Is A Buffer Value a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer has an effective ph range of one ph unit on either side of the. What Is A Buffer Value.
From www.slideserve.com
PPT Hydrolysis of Salts and pH of Buffer Solutions PowerPoint What Is A Buffer Value a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer has an effective ph range of. What Is A Buffer Value.
From 2012books.lardbucket.org
Buffers What Is A Buffer Value a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer solution is a solution where the ph does not. What Is A Buffer Value.
From morioh.com
Buffer Solutions What Is A Buffer Value a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. If the ph of a buffer goes out of this range, the buffer will no. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and. What Is A Buffer Value.