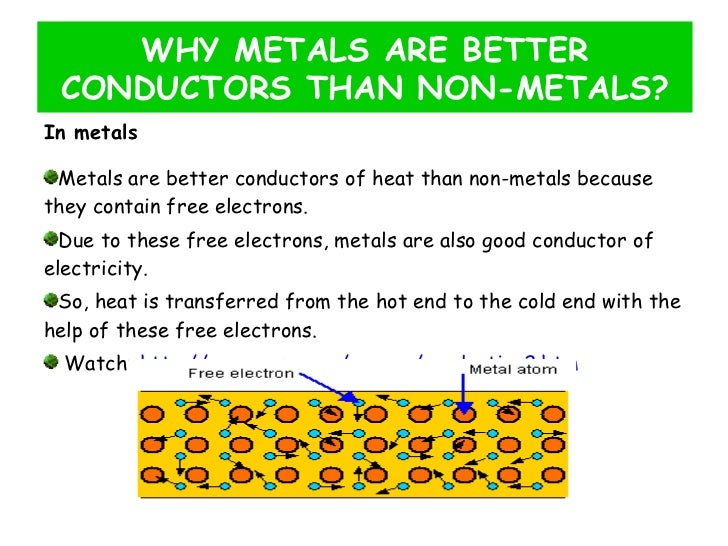
Metals Good Conductors Heat . When heat is applied, these electrons gain energy and. Metals are good conductors of heat and electricity because they contain a glut of free electrons. These free electrons are able to. Metals are usually crystalline solids. Metals are good conductors of heat because they have free electrons that can move easily. In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. In metals, some of the electrons (often one per atom) are not. Metals are highly important in making. What are some factors that influence a metal’s electrical conductivity? All metals can conduct heat and electric current, but some are more effective conductors than others. First, let me explain why metals generally conduct heat better than other solids do. This is due to the way that metals bond chemically:. Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy.
from www.slideshare.net
All metals can conduct heat and electric current, but some are more effective conductors than others. What are some factors that influence a metal’s electrical conductivity? Metals are good conductors of heat because they have free electrons that can move easily. In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. Metals are good conductors of heat and electricity because they contain a glut of free electrons. Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. Metals are highly important in making. In metals, some of the electrons (often one per atom) are not. When heat is applied, these electrons gain energy and. First, let me explain why metals generally conduct heat better than other solids do.
Chapter 24 Conduction
Metals Good Conductors Heat In metals, some of the electrons (often one per atom) are not. Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. Metals are good conductors of heat and electricity because they contain a glut of free electrons. These free electrons are able to. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. Metals are usually crystalline solids. Metals are highly important in making. First, let me explain why metals generally conduct heat better than other solids do. When heat is applied, these electrons gain energy and. All metals can conduct heat and electric current, but some are more effective conductors than others. This is due to the way that metals bond chemically:. What are some factors that influence a metal’s electrical conductivity? Metals are good conductors of heat because they have free electrons that can move easily. In metals, some of the electrons (often one per atom) are not.
From readership.org
Why Are Metals Good Conductors Of Both Heat And Electricity? Exploring The Physics Behind It Metals Good Conductors Heat This is due to the way that metals bond chemically:. Metals are good conductors of heat and electricity because they contain a glut of free electrons. All metals can conduct heat and electric current, but some are more effective conductors than others. When heat is applied, these electrons gain energy and. Metals are good conductors of heat because they have. Metals Good Conductors Heat.
From joiruwium.blob.core.windows.net
Copper Is A Good Conductor Of Electricity And Heat Justify The Statement at Marcia Rivera blog Metals Good Conductors Heat All metals can conduct heat and electric current, but some are more effective conductors than others. Metals are good conductors of heat because they have free electrons that can move easily. Metals are usually crystalline solids. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. Metals are good conductors of current and heat because of their. Metals Good Conductors Heat.
From exonhrnmo.blob.core.windows.net
Element Is The Best Conductor Of Heat And Electricity at Chris Rupe blog Metals Good Conductors Heat When heat is applied, these electrons gain energy and. In metals, some of the electrons (often one per atom) are not. What are some factors that influence a metal’s electrical conductivity? These free electrons are able to. First, let me explain why metals generally conduct heat better than other solids do. All metals can conduct heat and electric current, but. Metals Good Conductors Heat.
From seanrtmckee.blogspot.com
Good Conductor of Heat SeanrtMckee Metals Good Conductors Heat Metals are good conductors of heat because they have free electrons that can move easily. In metals, some of the electrons (often one per atom) are not. These free electrons are able to. Metals are highly important in making. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. First, let me explain why metals generally conduct. Metals Good Conductors Heat.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID1128267 Metals Good Conductors Heat All metals can conduct heat and electric current, but some are more effective conductors than others. In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. These free electrons are able to. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. This is. Metals Good Conductors Heat.
From www.universetoday.com
The Science of Heat Transfer What Is Conduction? Universe Today Metals Good Conductors Heat In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. All metals can conduct heat and electric current, but some are more effective conductors than others. What are some factors that influence a metal’s electrical conductivity? These free electrons are able to. Metals are good conductors of. Metals Good Conductors Heat.
From slideplayer.com
Periodic Table Chapter ppt download Metals Good Conductors Heat What are some factors that influence a metal’s electrical conductivity? Metals are good conductors of heat and electricity because they contain a glut of free electrons. Metals are usually crystalline solids. This is due to the way that metals bond chemically:. These free electrons are able to. In most cases, they have a relatively simple crystal structure distinguished by a. Metals Good Conductors Heat.
From klabnkmxn.blob.core.windows.net
Materials Are Good Conductors Of Heat And Electricity at Megan Fonseca blog Metals Good Conductors Heat Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. Metals are highly important in making. This is due to the way that metals bond chemically:. In metals, some of the electrons (often one per atom) are not. In most cases, they have a relatively simple crystal structure distinguished. Metals Good Conductors Heat.
From joikjmxpr.blob.core.windows.net
Name The Metal Which Is The Best Conductor Of Heat And Electricity at Troy Stewart blog Metals Good Conductors Heat Metals are usually crystalline solids. Metals are highly important in making. These free electrons are able to. What are some factors that influence a metal’s electrical conductivity? In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors. Metals Good Conductors Heat.
From slideplayer.com
HEAT. ppt download Metals Good Conductors Heat These free electrons are able to. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. When heat is applied, these electrons gain energy and. In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. Metals are good conductors of heat because they have. Metals Good Conductors Heat.
From slideplayer.com
Chapter 6 The periodic table ppt download Metals Good Conductors Heat In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. What are some factors that influence a metal’s electrical conductivity? Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. First, let me explain why metals generally conduct heat better than other solids do.. Metals Good Conductors Heat.
From www.youtube.com
Why metals are good conductors of heat ? YouTube Metals Good Conductors Heat Metals are usually crystalline solids. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. Metals are good conductors of heat because they have free electrons that can move easily. Metals are good conductors of heat and electricity because they contain a glut of free electrons. When heat is applied, these electrons gain energy and. In most. Metals Good Conductors Heat.
From joikjmxpr.blob.core.windows.net
Name The Metal Which Is The Best Conductor Of Heat And Electricity at Troy Stewart blog Metals Good Conductors Heat Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. Metals are usually crystalline solids. What are some factors that influence a metal’s electrical conductivity? Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. Metals are highly important in making. All metals can conduct heat. Metals Good Conductors Heat.
From www.ency123.com
Why are metals good conductors of heat and electricity? Metals Good Conductors Heat First, let me explain why metals generally conduct heat better than other solids do. What are some factors that influence a metal’s electrical conductivity? These free electrons are able to. In metals, some of the electrons (often one per atom) are not. In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and. Metals Good Conductors Heat.
From www.scienceabc.com
Why Are Metals Good Conductors Of Electricity And Heat? Metals Good Conductors Heat What are some factors that influence a metal’s electrical conductivity? Metals are good conductors of heat because they have free electrons that can move easily. All metals can conduct heat and electric current, but some are more effective conductors than others. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. Metals are good conductors of current. Metals Good Conductors Heat.
From www.slideshare.net
Chapter 24 Conduction Metals Good Conductors Heat These free electrons are able to. All metals can conduct heat and electric current, but some are more effective conductors than others. This is due to the way that metals bond chemically:. What are some factors that influence a metal’s electrical conductivity? When heat is applied, these electrons gain energy and. Metals are good conductors of current and heat because. Metals Good Conductors Heat.
From www.slideserve.com
PPT REVISION PowerPoint Presentation, free download ID307194 Metals Good Conductors Heat Metals are good conductors of heat because they have free electrons that can move easily. Metals are highly important in making. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. When heat is applied, these electrons gain energy and. What are some factors that influence a metal’s electrical conductivity? In metals, some of the electrons (often. Metals Good Conductors Heat.
From ar.inspiredpencil.com
Good Conductor Of Heat Metals Good Conductors Heat In metals, some of the electrons (often one per atom) are not. All metals can conduct heat and electric current, but some are more effective conductors than others. First, let me explain why metals generally conduct heat better than other solids do. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. These free electrons are able. Metals Good Conductors Heat.
From exonhrnmo.blob.core.windows.net
Element Is The Best Conductor Of Heat And Electricity at Chris Rupe blog Metals Good Conductors Heat Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. Metals are good conductors of heat because they have free electrons that can move easily. All metals can conduct heat and electric current, but some are more effective conductors than others. In most cases, they have a relatively simple crystal structure distinguished by a close packing of. Metals Good Conductors Heat.
From www.thoughtco.com
Conductivity and Conductive Elements Metals Good Conductors Heat When heat is applied, these electrons gain energy and. Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. Metals are good conductors of heat and electricity because they contain a glut of free electrons. First, let me explain why metals generally conduct heat better than other solids do.. Metals Good Conductors Heat.
From www.slideserve.com
PPT AQA GCSE 1a1 Heat Transfer PowerPoint Presentation, free download ID281065 Metals Good Conductors Heat Metals are good conductors of heat because they have free electrons that can move easily. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. This is due to the way that metals bond chemically:. Metals are highly important in making. All metals can conduct heat and electric current, but some are more effective conductors than others.. Metals Good Conductors Heat.
From www.gkseries.com
Metals are good conductors of heat because Metals Good Conductors Heat Metals are good conductors of heat because they have free electrons that can move easily. This is due to the way that metals bond chemically:. Metals are highly important in making. Metals are good conductors of heat and electricity because they contain a glut of free electrons. What are some factors that influence a metal’s electrical conductivity? All metals can. Metals Good Conductors Heat.
From slidetodoc.com
Metals 1 Metals are good conductors of heat Metals Good Conductors Heat All metals can conduct heat and electric current, but some are more effective conductors than others. Metals are good conductors of heat because they have free electrons that can move easily. What are some factors that influence a metal’s electrical conductivity? This is due to the way that metals bond chemically:. Metals (e.g., copper, platinum, gold, etc.) are usually good. Metals Good Conductors Heat.
From www.samaterials.com
What Metal is a Good Conductor of Heat Metals Good Conductors Heat These free electrons are able to. Metals are good conductors of heat because they have free electrons that can move easily. Metals are highly important in making. In metals, some of the electrons (often one per atom) are not. In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree. Metals Good Conductors Heat.
From thevigyan.com
Why Are Metals Good Conductors of Heat? The Vigyan Metals Good Conductors Heat All metals can conduct heat and electric current, but some are more effective conductors than others. Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. Metals are good conductors of heat and electricity because they contain a glut of free electrons. This is due to the way that. Metals Good Conductors Heat.
From www.youtube.com
Metals good conductors of heat.mov YouTube Metals Good Conductors Heat All metals can conduct heat and electric current, but some are more effective conductors than others. First, let me explain why metals generally conduct heat better than other solids do. These free electrons are able to. In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. This. Metals Good Conductors Heat.
From www.thoughtco.com
Electrical Conductivity of Metals Metals Good Conductors Heat First, let me explain why metals generally conduct heat better than other solids do. Metals are good conductors of heat and electricity because they contain a glut of free electrons. What are some factors that influence a metal’s electrical conductivity? Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. Metals are good conductors of current and. Metals Good Conductors Heat.
From dxohinwej.blob.core.windows.net
Name The Metal Which Is The Best Conductor Of Heat at Jeanne Buckle blog Metals Good Conductors Heat Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. All metals can conduct heat and electric current, but some are more effective conductors than others. Metals are good conductors of heat and electricity because they contain a glut of free electrons. Metals are good conductors of heat because they have free electrons that can move easily.. Metals Good Conductors Heat.
From www.youtube.com
Metals are Good Conductor of Heat NCERT Class 10th Lab Activity YouTube Metals Good Conductors Heat In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. Metals are good conductors of heat because they have free electrons that can move easily. When heat is applied, these electrons gain energy and. First, let me explain why metals generally conduct heat better than other solids. Metals Good Conductors Heat.
From www.aiophotoz.com
What Are Conductors And Insulators Examples Of Conductors And Images and Photos finder Metals Good Conductors Heat Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. This is due to the way that metals bond chemically:. Metals are good conductors of heat because they have free electrons that can move easily. These free electrons are able to. First, let me explain why metals generally conduct. Metals Good Conductors Heat.
From www.youtube.com
CONDUCTORS AND INSULATORS SCIENCE EDUCATIONAL VIDEO FOR KIDS YouTube Metals Good Conductors Heat When heat is applied, these electrons gain energy and. In metals, some of the electrons (often one per atom) are not. First, let me explain why metals generally conduct heat better than other solids do. What are some factors that influence a metal’s electrical conductivity? All metals can conduct heat and electric current, but some are more effective conductors than. Metals Good Conductors Heat.
From www.youtube.com
To Show that Metals are Good Conductors of Heat and Have High Melting Point YouTube Metals Good Conductors Heat What are some factors that influence a metal’s electrical conductivity? Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. When heat is applied, these electrons gain energy and. This is due to the way that. Metals Good Conductors Heat.
From techiescientist.com
Does Metal Conduct Heat? (Detailed Explanation) Techiescientist Metals Good Conductors Heat Metals are highly important in making. When heat is applied, these electrons gain energy and. These free electrons are able to. All metals can conduct heat and electric current, but some are more effective conductors than others. Metals are good conductors of heat and electricity because they contain a glut of free electrons. What are some factors that influence a. Metals Good Conductors Heat.
From www.slideshare.net
Conduction Lesson 2 Metals Good Conductors Heat This is due to the way that metals bond chemically:. All metals can conduct heat and electric current, but some are more effective conductors than others. Metals are usually crystalline solids. In most cases, they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry. Metals (e.g., copper, platinum, gold, etc.). Metals Good Conductors Heat.
From dxohinwej.blob.core.windows.net
Name The Metal Which Is The Best Conductor Of Heat at Jeanne Buckle blog Metals Good Conductors Heat Metals are good conductors of current and heat because of their metallic bonding which provides a large number of free electrons. First, let me explain why metals generally conduct heat better than other solids do. Metals are highly important in making. Metals are usually crystalline solids. Metals (e.g., copper, platinum, gold, etc.) are usually good conductors of thermal energy. What. Metals Good Conductors Heat.