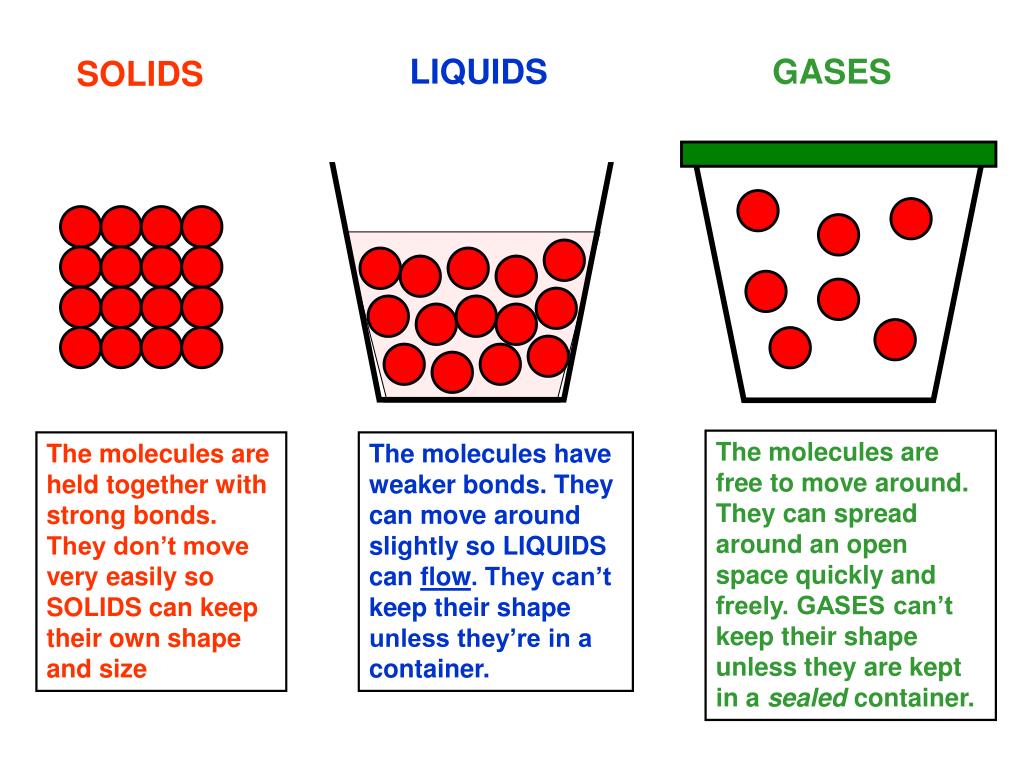
Solids And Liquids In Reaction Quotients . The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Working out final equilibrium concentration from any starting position. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. When dealing with these equilibria, remember that solids and pure liquids do not appear in. Equilibria involving liquids and solids. Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant. The phases may be any combination of solid, liquid, or gas phases, and solutions. Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams.
from www.slideserve.com
Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. The phases may be any combination of solid, liquid, or gas phases, and solutions. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. When dealing with these equilibria, remember that solids and pure liquids do not appear in. Working out final equilibrium concentration from any starting position. Equilibria involving liquids and solids. Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant.
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID
Solids And Liquids In Reaction Quotients Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant. Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant. Working out final equilibrium concentration from any starting position. The phases may be any combination of solid, liquid, or gas phases, and solutions. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. Equilibria involving liquids and solids. When dealing with these equilibria, remember that solids and pure liquids do not appear in. Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the.
From general.chemistrysteps.com
Reaction Quotient Q Chemistry Steps Solids And Liquids In Reaction Quotients The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. Working out final equilibrium concentration from any starting position. Equilibria involving liquids and solids. Solids and. Solids And Liquids In Reaction Quotients.
From studylib.net
I. The Reaction Quotient Solids And Liquids In Reaction Quotients Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. The phases may be any combination of solid, liquid, or gas phases, and solutions. The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Equilibria involving liquids and solids. When. Solids And Liquids In Reaction Quotients.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Solids And Liquids In Reaction Quotients The phases may be any combination of solid, liquid, or gas phases, and solutions. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a. Solids And Liquids In Reaction Quotients.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Solids And Liquids In Reaction Quotients Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant. Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Equilibria involving liquids and solids.. Solids And Liquids In Reaction Quotients.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube Solids And Liquids In Reaction Quotients Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Working out final equilibrium concentration from any starting position. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Solid and pure liquids. Solids And Liquids In Reaction Quotients.
From www.youtube.com
Reaction Quotient Q Lecture and Example (Pt. 7) YouTube Solids And Liquids In Reaction Quotients Working out final equilibrium concentration from any starting position. When dealing with these equilibria, remember that solids and pure liquids do not appear in. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. The reaction quotient is equal to the molar concentrations of the. Solids And Liquids In Reaction Quotients.
From www.numerade.com
SOLVEDWrite an equilibrium expression for each chemical equation Solids And Liquids In Reaction Quotients When dealing with these equilibria, remember that solids and pure liquids do not appear in. Working out final equilibrium concentration from any starting position. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. Solids and liquids, as long as there is some available,. Solids And Liquids In Reaction Quotients.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solids And Liquids In Reaction Quotients The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Equilibria involving liquids and solids. Working out final equilibrium concentration from any starting position. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. Solid and. Solids And Liquids In Reaction Quotients.
From byjus.com
5.How found the reaction quotient (Q) of a cell? Solids And Liquids In Reaction Quotients Working out final equilibrium concentration from any starting position. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. When dealing with these equilibria, remember that solids and pure liquids do not appear in. Study guides on the reaction quotient for the college board. Solids And Liquids In Reaction Quotients.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2395087 Solids And Liquids In Reaction Quotients When dealing with these equilibria, remember that solids and pure liquids do not appear in. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Study. Solids And Liquids In Reaction Quotients.
From www.snexplores.org
Explainer What are the different states of matter? Solids And Liquids In Reaction Quotients Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Working out final equilibrium concentration from any starting position. When dealing with these equilibria, remember that solids and pure liquids do not appear in. The phases may be any combination of solid, liquid, or gas. Solids And Liquids In Reaction Quotients.
From grigerscience.weebly.com
Properties of Matter Griger Science Solids And Liquids In Reaction Quotients Working out final equilibrium concentration from any starting position. Equilibria involving liquids and solids. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. When dealing with these equilibria, remember that solids and pure liquids do not appear in. The reaction quotient (\(q\)) measures. Solids And Liquids In Reaction Quotients.
From www.slideserve.com
PPT Unit 2 Liquids & solids, solubility, equilibrium PowerPoint Solids And Liquids In Reaction Quotients Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. The. Solids And Liquids In Reaction Quotients.
From saylordotorg.github.io
Solids and Liquids Solids And Liquids In Reaction Quotients Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. The phases may be any combination of solid, liquid, or gas phases, and solutions. Working out final equilibrium concentration from any starting position. Solids and liquids, as long as there is some available, are fully available to the. Solids And Liquids In Reaction Quotients.
From slideplayer.com
PCAT Prep Chemistry Section ppt download Solids And Liquids In Reaction Quotients The phases may be any combination of solid, liquid, or gas phases, and solutions. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. Working out final equilibrium concentration from any starting position. The reaction quotient (\(q\)) measures the relative amounts of products and. Solids And Liquids In Reaction Quotients.
From www.slideserve.com
PPT Solid State Reactions PowerPoint Presentation, free download ID Solids And Liquids In Reaction Quotients Equilibria involving liquids and solids. The phases may be any combination of solid, liquid, or gas phases, and solutions. Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant. The reaction quotient is equal to the molar. Solids And Liquids In Reaction Quotients.
From study.com
Equilibrium Constant & Reaction Quotient Calculation & Examples Solids And Liquids In Reaction Quotients Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. Equilibria involving liquids and solids. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. The reaction quotient (\(q\)) measures the relative amounts. Solids And Liquids In Reaction Quotients.
From slideplayer.com
Lecture PowerPoint Chemistry The Molecular Nature of Matter and Change Solids And Liquids In Reaction Quotients Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. When dealing with these equilibria, remember that solids and pure liquids do not appear in. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not. Solids And Liquids In Reaction Quotients.
From www.slideserve.com
PPT Reaction Quotient PowerPoint Presentation ID5084895 Solids And Liquids In Reaction Quotients Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant. Working out final equilibrium concentration from any starting position. Equilibria involving liquids and solids. When dealing with these equilibria, remember that solids and pure liquids do not. Solids And Liquids In Reaction Quotients.
From slideplayer.com
Thermodynamics, pt 2 Dr. Harris Lecture 22 (Ch 23) 11/19/12 ppt download Solids And Liquids In Reaction Quotients The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry. Solids And Liquids In Reaction Quotients.
From slideplayer.com
The Nernst Equation. ppt download Solids And Liquids In Reaction Quotients Working out final equilibrium concentration from any starting position. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. Equilibria involving liquids and solids. The phases may be any combination of solid, liquid, or gas phases, and solutions. Study guides on the reaction quotient. Solids And Liquids In Reaction Quotients.
From slideplayer.com
CHAPTER 15 Chemical Equilibrium. ppt download Solids And Liquids In Reaction Quotients Working out final equilibrium concentration from any starting position. When dealing with these equilibria, remember that solids and pure liquids do not appear in. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. The reaction quotient is equal to the molar concentrations of the. Solids And Liquids In Reaction Quotients.
From slideplayer.com
Chemical Equilibrium. ppt download Solids And Liquids In Reaction Quotients When dealing with these equilibria, remember that solids and pure liquids do not appear in. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as. Solids And Liquids In Reaction Quotients.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2395087 Solids And Liquids In Reaction Quotients The phases may be any combination of solid, liquid, or gas phases, and solutions. Working out final equilibrium concentration from any starting position. When dealing with these equilibria, remember that solids and pure liquids do not appear in. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does. Solids And Liquids In Reaction Quotients.
From www.slideshare.net
Chemical equilibrium Solids And Liquids In Reaction Quotients When dealing with these equilibria, remember that solids and pure liquids do not appear in. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save. Solids And Liquids In Reaction Quotients.
From www.youtube.com
Chemical Equilibrium 4 Reaction Quotient 5m35s YouTube Solids And Liquids In Reaction Quotients The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. The phases may be any combination of solid, liquid, or gas phases, and solutions. Equilibria involving. Solids And Liquids In Reaction Quotients.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Solids And Liquids In Reaction Quotients The phases may be any combination of solid, liquid, or gas phases, and solutions. The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Study guides on. Solids And Liquids In Reaction Quotients.
From www.youtube.com
Chemical Equilibrium Reaction Quotient & Application of a Large K Solids And Liquids In Reaction Quotients Working out final equilibrium concentration from any starting position. The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. When dealing with these equilibria, remember that. Solids And Liquids In Reaction Quotients.
From www.showme.com
Reaction Quotient Chemistry equilibrium ShowMe Solids And Liquids In Reaction Quotients Equilibria involving liquids and solids. Working out final equilibrium concentration from any starting position. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save. Solids And Liquids In Reaction Quotients.
From zakruti.com
Using the reaction quotient Equilibrium AP Chemistry Solids And Liquids In Reaction Quotients Working out final equilibrium concentration from any starting position. The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Study guides on the reaction quotient for. Solids And Liquids In Reaction Quotients.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Solids And Liquids In Reaction Quotients Working out final equilibrium concentration from any starting position. The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. The reaction quotient is equal to the molar. Solids And Liquids In Reaction Quotients.
From slideplayer.com
Equilibrium Constant in Terms of Pressure, Heterogeneous Equilibria Solids And Liquids In Reaction Quotients Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant. Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. The reaction quotient (\(q\)) measures. Solids And Liquids In Reaction Quotients.
From www.youtube.com
Reaction Quotients YouTube Solids And Liquids In Reaction Quotients The reaction quotient is equal to the molar concentrations of the products of the chemical equation (multiplied together) over the reactants (also multiplied together), with each. Working out final equilibrium concentration from any starting position. The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. The phases may be any combination of. Solids And Liquids In Reaction Quotients.
From www.slideserve.com
PPT Ionic Equilibria III The Solubility Product Principle PowerPoint Solids And Liquids In Reaction Quotients The reaction quotient (\(q\)) measures the relative amounts of products and reactants present during a reaction at a. Solid and pure liquids (not solutions) have constant densities and therefore their concentrations are constant and do not change as a reaction proceeds and thus they are not kept in the equilibrium constant. Working out final equilibrium concentration from any starting position.. Solids And Liquids In Reaction Quotients.
From www.youtube.com
15.5 Heterogeneous Equilibria Reactions Involving a Solid or a Liquid Solids And Liquids In Reaction Quotients Working out final equilibrium concentration from any starting position. Study guides on the reaction quotient for the college board ap® chemistry syllabus, written by the chemistry experts at save my exams. The phases may be any combination of solid, liquid, or gas phases, and solutions. When dealing with these equilibria, remember that solids and pure liquids do not appear in.. Solids And Liquids In Reaction Quotients.