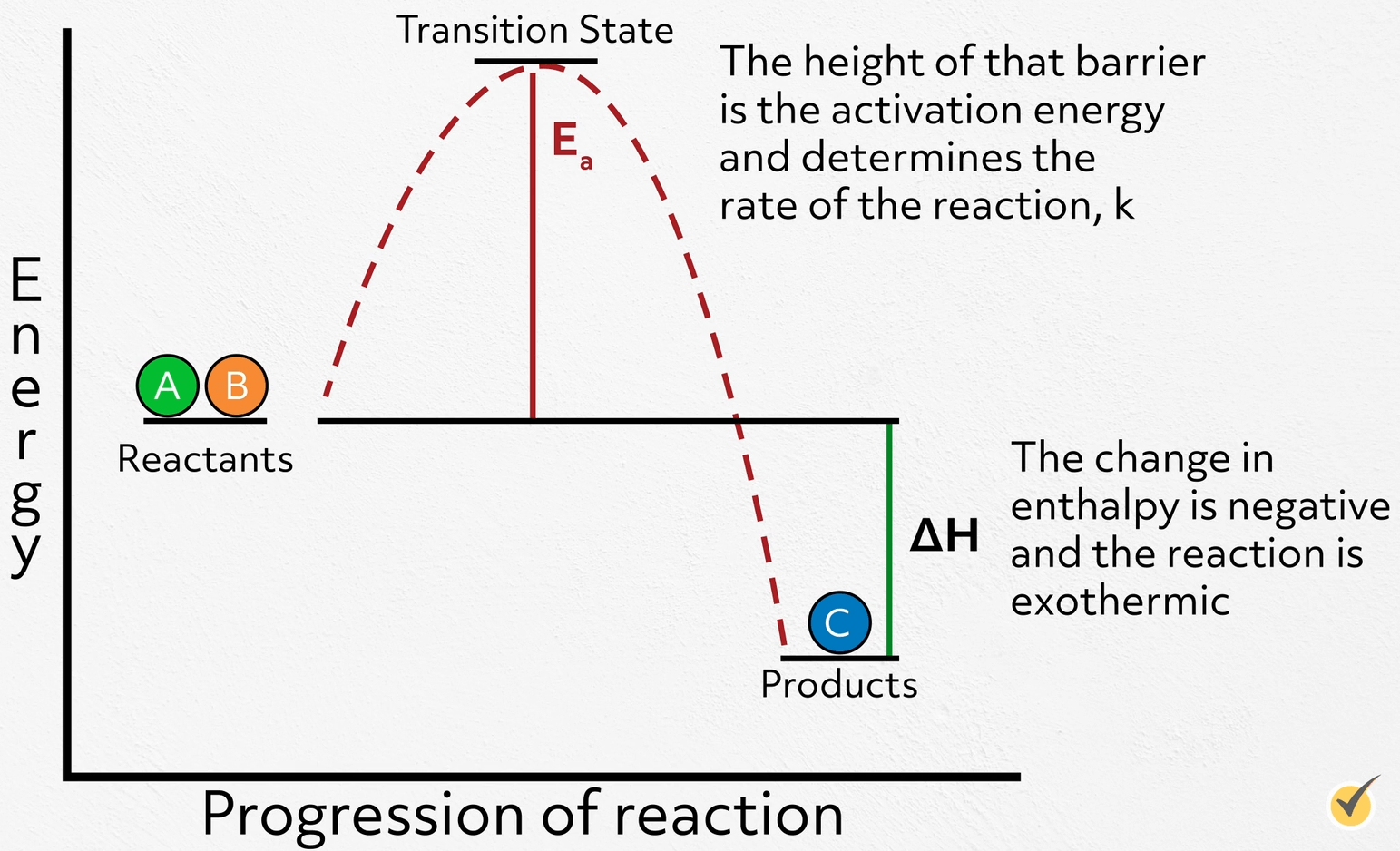
Catalyst Reaction Formula . What are catalysts, and how do they work in terms altering the parameters of a reaction? They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts can be homogenous (in the same phase as the reactants) or. Describe the similarities and differences between the three principal classes of catalysts. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. By the end of this section, you will be able to: In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Explain the function of a catalyst in terms of reaction mechanisms and potential. Catalysts allow a reaction to proceed via a. It is not consumed by the process. By the end of this section, you will be able to:. Catalysts participate in a chemical reaction and increase its rate.
from www.mometrix.com
By the end of this section, you will be able to: Describe the similarities and differences between the three principal classes of catalysts. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net equation and are not consumed during the reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. By the end of this section, you will be able to:. Catalysts allow a reaction to proceed via a. Catalysts can be homogenous (in the same phase as the reactants) or. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts participate in a chemical reaction and increase its rate.
What is a Catalyst? Chemistry Review (Video)
Catalyst Reaction Formula It is not consumed by the process. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It is not consumed by the process. Catalysts allow a reaction to proceed via a. By the end of this section, you will be able to: By the end of this section, you will be able to:. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal classes of catalysts. Explain the function of a catalyst in terms of reaction mechanisms and potential. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog Catalyst Reaction Formula Explain the function of a catalyst in terms of reaction mechanisms and potential. What are catalysts, and how do they work in terms altering the parameters of a reaction? By the end of this section, you will be able to:. Catalysts allow a reaction to proceed via a. By the end of this section, you will be able to: Catalysts. Catalyst Reaction Formula.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Reaction Formula Catalysts can be homogenous (in the same phase as the reactants) or. By the end of this section, you will be able to:. By the end of this section, you will be able to: A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide. Catalyst Reaction Formula.
From 2012books.lardbucket.org
Catalysis Catalyst Reaction Formula By the end of this section, you will be able to:. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts can be homogenous (in the same phase as the reactants). Catalyst Reaction Formula.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Reaction Formula They do not appear in the reaction’s net equation and are not consumed during the reaction. It is not consumed by the process. Describe the similarities and differences between the three principal classes of catalysts. Catalysts participate in a chemical reaction and increase its rate. By the end of this section, you will be able to:. By the end of. Catalyst Reaction Formula.
From www.slideserve.com
PPT ENZYME BIOLOGICAL CATALYST PowerPoint Presentation, free Catalyst Reaction Formula They do not appear in the reaction’s net equation and are not consumed during the reaction. By the end of this section, you will be able to: Catalysts allow a reaction to proceed via a. Explain the function of a catalyst in terms of reaction mechanisms and potential. Catalysts participate in a chemical reaction and increase its rate. What are. Catalyst Reaction Formula.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalyst Reaction Formula By the end of this section, you will be able to:. It is not consumed by the process. Describe the similarities and differences between the three principal classes of catalysts. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst lowers the activation energy of a reaction, increasing its rate. What are. Catalyst Reaction Formula.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalyst Reaction Formula Describe the similarities and differences between the three principal classes of catalysts. Explain the function of a catalyst in terms of reaction mechanisms and potential. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a. By the end of this section, you will be able to:. Catalyst Reaction Formula.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Reaction Formula Explain the function of a catalyst in terms of reaction mechanisms and potential. They do not appear in the reaction’s net equation and are not consumed during the reaction. By the end of this section, you will be able to: In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed. Catalyst Reaction Formula.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Reaction Formula Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same phase as the reactants) or. A catalyst lowers the activation energy of a reaction, increasing its rate. By the end of this section, you will be able to: By the end of this section, you. Catalyst Reaction Formula.
From ar.inspiredpencil.com
Catalyst Equation Catalyst Reaction Formula In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Describe the similarities and differences between the three principal classes of catalysts. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Explain the function of a catalyst in terms. Catalyst Reaction Formula.
From www.numerade.com
SOLVEDA catalyst lowers the activation energy of a reaction from 215 Catalyst Reaction Formula Catalysts participate in a chemical reaction and increase its rate. It is not consumed by the process. Describe the similarities and differences between the three principal classes of catalysts. By the end of this section, you will be able to: Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do. Catalyst Reaction Formula.
From slideplayer.com
Chemical Reactions. ppt download Catalyst Reaction Formula It is not consumed by the process. A catalyst lowers the activation energy of a reaction, increasing its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a. Describe the similarities and differences between the three principal classes of catalysts. What are catalysts, and how. Catalyst Reaction Formula.
From www.essentialchemicalindustry.org
Catalysis in industry Catalyst Reaction Formula Catalysts can be homogenous (in the same phase as the reactants) or. They do not appear in the reaction’s net equation and are not consumed during the reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential. Catalysts participate in a chemical reaction and increase its rate. Describe the similarities and differences between the three principal. Catalyst Reaction Formula.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalyst Reaction Formula A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. What are catalysts, and how do they work in terms altering the parameters of a reaction? By the end of this section, you will be able to:. Catalysts allow a. Catalyst Reaction Formula.
From www.slideserve.com
PPT Reaction Mechanism PowerPoint Presentation, free download ID Catalyst Reaction Formula It is not consumed by the process. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. They do not appear in the reaction’s net equation and are not consumed during the reaction. By the end of this section, you will be able to: A catalyst lowers the. Catalyst Reaction Formula.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalyst Reaction Formula Catalysts can be homogenous (in the same phase as the reactants) or. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It is not consumed by the process. Catalysts allow a reaction to proceed via. Catalyst Reaction Formula.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalyst Reaction Formula A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It is not consumed by the process. By. Catalyst Reaction Formula.
From commons.wikimedia.org
FileCatalitic cycle for hydrogenation with Wilkinson's catalyst.svg Catalyst Reaction Formula Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts participate in a chemical reaction and increase its rate. Describe the similarities and differences between the three principal classes of catalysts. By the end of this section, you will be able to:. It is not consumed by the process. Explain the function of a catalyst in terms. Catalyst Reaction Formula.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Reaction Formula Catalysts participate in a chemical reaction and increase its rate. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Describe the similarities and differences between the three principal classes of catalysts. A catalyst lowers the activation energy of a reaction, increasing its rate. By the end of. Catalyst Reaction Formula.
From www.sitemap.odinity.com
Synthesis and Studies of Wilkinson’s Catalyst Odinity Catalyst Reaction Formula Catalysts allow a reaction to proceed via a. By the end of this section, you will be able to:. They do not appear in the reaction’s net equation and are not consumed during the reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Describe the similarities. Catalyst Reaction Formula.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Catalyst Reaction Formula It is not consumed by the process. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. By the end of this section, you will be able to:. Catalysts participate in a chemical reaction and increase its rate. What are catalysts, and how do they work in terms. Catalyst Reaction Formula.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID3206932 Catalyst Reaction Formula A catalyst lowers the activation energy of a reaction, increasing its rate. Explain the function of a catalyst in terms of reaction mechanisms and potential. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. By the end of this section, you will be able to:. What are catalysts, and how do. Catalyst Reaction Formula.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Reaction Formula By the end of this section, you will be able to: Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to proceed via a. Explain the function of a catalyst in terms of reaction mechanisms and potential. Describe the similarities and differences between. Catalyst Reaction Formula.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Reaction Formula Explain the function of a catalyst in terms of reaction mechanisms and potential. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. They do not appear in the reaction’s net equation and are not consumed during the reaction. Describe the similarities and differences between the three principal classes of catalysts. Catalysts. Catalyst Reaction Formula.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Reaction Formula A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. What are catalysts, and how do they work in terms altering the parameters of a reaction? It is not consumed by the process. In chemistry and biology, a catalyst is. Catalyst Reaction Formula.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Reaction Formula They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts participate in a chemical reaction and increase its rate. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. In chemistry and biology,. Catalyst Reaction Formula.
From ar.inspiredpencil.com
Catalyst Reaction Diagram Catalyst Reaction Formula Catalysts participate in a chemical reaction and increase its rate. It is not consumed by the process. Explain the function of a catalyst in terms of reaction mechanisms and potential. By the end of this section, you will be able to: They do not appear in the reaction’s net equation and are not consumed during the reaction. Describe the similarities. Catalyst Reaction Formula.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalyst Reaction Formula By the end of this section, you will be able to:. Describe the similarities and differences between the three principal classes of catalysts. It is not consumed by the process. Catalysts participate in a chemical reaction and increase its rate. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts allow a reaction to proceed via a.. Catalyst Reaction Formula.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst Reaction Formula They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. A catalyst lowers the activation energy of a reaction, increasing its rate. Explain the function of a catalyst in terms of reaction mechanisms and potential. In chemistry and biology, a catalyst is a substance. Catalyst Reaction Formula.
From joilxpqmt.blob.core.windows.net
Chemical Catalysis Examples at Howard Wade blog Catalyst Reaction Formula In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It is not consumed by the process. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts. Catalyst Reaction Formula.
From hubpages.com
Chemical Reactions and Chemical Equations Owlcation Catalyst Reaction Formula A catalyst lowers the activation energy of a reaction, increasing its rate. Describe the similarities and differences between the three principal classes of catalysts. It is not consumed by the process. Catalysts participate in a chemical reaction and increase its rate. Catalysts can be homogenous (in the same phase as the reactants) or. They do not appear in the reaction’s. Catalyst Reaction Formula.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalyst Reaction Formula Catalysts allow a reaction to proceed via a. Catalysts participate in a chemical reaction and increase its rate. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts can be homogenous (in the same phase. Catalyst Reaction Formula.
From www.youtube.com
Catalysts Chemistry Khan Academy YouTube Catalyst Reaction Formula Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. By the end of this section, you will be able to: What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal classes of catalysts. It is not. Catalyst Reaction Formula.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID3650426 Catalyst Reaction Formula A catalyst lowers the activation energy of a reaction, increasing its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. It is not consumed by the process. What are catalysts,. Catalyst Reaction Formula.
From www.numerade.com
SOLVED write a chemical reaction which is carried out by catalyst with Catalyst Reaction Formula Explain the function of a catalyst in terms of reaction mechanisms and potential. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. By the end of this section, you will be able to:. Catalysts allow a reaction to proceed via a. It is not consumed by the process. In chemistry and. Catalyst Reaction Formula.